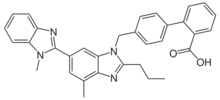
Chemistry:Telmisartan
 | |
| Clinical data | |
|---|---|
| Pronunciation | /tɛlmɪˈsɑːrtən/ |
| Trade names | Micardis, Actavis, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601249 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Angiotensin II receptor antagonist |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 42–100% |
| Protein binding | >99.5% |
| Metabolism | Minimal liver (glucuronidation) |
| Elimination half-life | 24 hours |
| Excretion | Feces 97% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C33H30N4O2 |
| Molar mass | 514.629 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Telmisartan, sold under the brand name Micardis among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1] It is taken by mouth.[1] Versions are available as the combination[2] telmisartan/hydrochlorothiazide, telmisartan/cilnidipine[3] and telmisartan/amlodipine.[1]
Common side effects include upper respiratory tract infections, diarrhea, and back pain.[1] Serious side effects may include kidney problems, low blood pressure, and angioedema.[1] Use in pregnancy may harm the baby and use when breastfeeding is not recommended.[4] It is an angiotensin II receptor antagonist and works by blocking the effects of angiotensin II.[1]
Telmisartan was patented in 1991 and came into medical use in 1999.[5] It is available as a generic medication.[6] In 2021, it was the 217th most commonly prescribed medication in the United States, with about 2 million prescriptions.[7]
Medical uses
Telmisartan is used to treat high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1][8]:146
Contraindications
Telmisartan is contraindicated during pregnancy. Like other drugs affecting the renin–angiotensin system (RAS), telmisartan can cause birth defects, stillbirths, and neonatal deaths. It is not known whether the drug passes into the breast milk.[9] Also it is contraindicated in bilateral renal artery stenosis in which it can cause kidney failure.
Side effects
Side effects are similar to other angiotensin II receptor antagonists and include tachycardia and bradycardia (fast or slow heartbeat), hypotension (low blood pressure) and edema (swelling of arms, legs, lips, tongue, or throat, the latter leading to breathing problems). Allergic reactions may also occur.[9]
Interactions
Due to its mechanism of action, telmisartan increases blood potassium levels. Combination with potassium preparations or potassium-sparing diuretics could cause hyperkalaemia (excessive potassium levels). Combination with NSAIDs, especially in patients with impaired kidney function, has a risk of causing (usually reversible) kidney failure.[10]
Pharmacology
Mechanism of action
Telmisartan is an angiotensin II receptor blocker that shows high affinity for the angiotensin II receptor type 1 (AT1), with a binding affinity 3000 times greater for AT1 than AT2.
In addition to blocking the renin–angiotensin system, telmisartan acts as a selective modulator of peroxisome proliferator-activated receptor gamma (PPAR-γ), a central regulator of insulin and glucose metabolism. It is believed that telmisartan's dual mode of action may provide protective benefits against the vascular and renal damage caused by diabetes and cardiovascular disease (CVD).[11]
Telmisartan's activity at the peroxisome proliferator-activated receptor delta (PPAR-δ) receptor has prompted speculation around its potential as a sport doping agent as an alternative to GW 501516.[12] Telmisartan activates PPAR-δ receptors in several tissues.[13][14][15][16]
Also, telmisartan has a PPAR-γ agonist activity.[8]:171
Pharmacokinetics
The substance is quickly but to varying degrees absorbed from the gut. The average bioavailability is about 50% (42–100%). Food intake has no clinically relevant influence on the kinetics of telmisartan. Plasma protein binding is over 99.5%, mainly to albumin and alpha-1-acid glycoprotein.[10] It has the longest half-life of any angiotensin II receptor blocker (ARB) (24 hours)[17][11] and the largest volume of distribution among ARBs (500 liters).[18][19] Less than 3% of telmisartan is inactivated by glucuronidation in the liver, and over 97% is eliminated in unchanged form via bile and faeces.[1][10]
History
Society and culture
Telmisartan is available as a generic medication.[6]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Telmisartan Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/telmisartan.html.
- ↑ "Formulation and Evaluation of Mouth Dissolving Tablets of Telmisartan". http://inventi.in/journal/article/rapid/2/28615/ndds/h.[yes|permanent dead link|dead link}}]
- ↑ "Cilacar T". Medical Dialogues. https://medicaldialogues.in/partner/jbcpl/cilacar-t-cilnidipine-telmisartan.
- ↑ "Telmisartan Pregnancy and Breastfeeding Warnings" (in en). https://www.drugs.com/pregnancy/telmisartan.html.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 471. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA471.
- ↑ 6.0 6.1 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 178. ISBN 9780857113382.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ 8.0 8.1 Drugs for the heart (seventh ed.). Saunders. 2009. ISBN 978-1-4160-6158-8.
- ↑ 9.0 9.1 Drugs.com: Micardis
- ↑ 10.0 10.1 10.2 Haberfeld, H, ed (2015) (in de). Austria-Codex. Vienna: Österreichischer Apothekerverlag.
- ↑ 11.0 11.1 "Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity". Hypertension 43 (5): 993–1002. May 2004. doi:10.1161/01.HYP.0000123072.34629.57. PMID 15007034.
- ↑ "Telmisartan as metabolic modulator: a new perspective in sports doping?". Journal of Strength and Conditioning Research 26 (3): 608–10. March 2012. doi:10.1519/JSC.0b013e31824301b6. PMID 22130396.
- ↑ Cytoplasmic and Nuclear Receptors: Advances in Research and Application: 2011 Edition. ScholarlyEditions. 2012. pp. 21–. ISBN 978-1-464-93110-9. https://books.google.com/books?id=9qCMLa1sIN0C&pg=PA21. Retrieved 2 April 2013.
- ↑ "Angiotensin II receptor blocker telmisartan enhances running endurance of skeletal muscle through activation of the PPAR-δ/AMPK pathway". Journal of Cellular and Molecular Medicine 15 (7): 1572–81. July 2011. doi:10.1111/j.1582-4934.2010.01085.x. PMID 20477906.
- ↑ "Telmisartan prevents weight gain and obesity through activation of peroxisome proliferator-activated receptor-delta-dependent pathways". Hypertension 55 (4): 869–79. April 2010. doi:10.1161/HYPERTENSIONAHA.109.143958. PMID 20176998.
- ↑ "Telmisartan improves insulin resistance of skeletal muscle through peroxisome proliferator-activated receptor-δ activation". Diabetes 62 (3): 762–74. March 2013. doi:10.2337/db12-0570. PMID 23238297.
- ↑ Pritor prescribing information
- ↑ "Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients". The Journal of International Medical Research 28 (4): 149–67. 2000. doi:10.1177/147323000002800401. PMID 11014323.
- ↑ "A review of telmisartan in the treatment of hypertension: blood pressure control in the early morning hours". Vascular Health and Risk Management 2 (3): 195–201. September 2006. doi:10.2147/vhrm.2006.2.3.195. PMID 17326326.
Further reading
- "Telmisartan, ramipril, or both in patients at high risk for vascular events". The New England Journal of Medicine (Massachusetts Medical Society) 358 (15): 1547–59. April 2008. doi:10.1056/nejmoa0801317. PMID 18378520.
- "Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial". Lancet 372 (9644): 1174–83. September 2008. doi:10.1016/S0140-6736(08)61242-8. PMID 18757085. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(08)61242-8/abstract. Retrieved 26 November 2019.
External links
- "Telmisartan". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/telmisartan.
 |

