Chemistry:Praseodymium(III) iodide
From HandWiki
| |
| Names | |
|---|---|
| Other names
Praseodymium triiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| PrI3 | |
| Molar mass | 521.619 g/mol 683.75652 g/mol (nonahydrate) |
| Appearance | hygroscopic green crystals |
| Density | 5.8 g/cm3[1] |
| Melting point | 738 °C (1,360 °F; 1,011 K)[1] |
| Boiling point | 1,380 °C (2,520 °F; 1,650 K) |
| 213.9 g/100 mL[2] | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H317, H360 | |
| P203Script error: No such module "Preview warning".Category:GHS errors, P261, P272, P280, P302+352, P318Script error: No such module "Preview warning".Category:GHS errors, P321, P333+313, P362+364Script error: No such module "Preview warning".Category:GHS errors, P405, P501 | |
| Related compounds | |
Other anions
|
Praseodymium(III) fluoride Praseodymium(III) chloride Praseodymium(III) bromide |
Other cations
|
Cerium(III) iodide Neodymium(III) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium and iodine, with the chemical formula PrI3. It forms green crystals.[4] It is soluble in water.[5]
Preparation
- [math]\displaystyle{ \mathsf{2Pr + 3I_2 \ \xrightarrow{T}\ 2PrI_3} }[/math]
- It can also be obtained by heating praseodymium with mercury(II) iodide:[6]
- [math]\displaystyle{ \mathsf{2Pr + 3HgI_2 \ \xrightarrow{T}\ 2PrI_3 + 3Hg} }[/math]
Properties
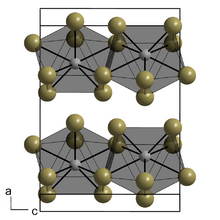
Praseodymium(III) iodide forms green crystals, which are soluble in water.[5] It forms orthorhombic crystals which are hygroscopic.[1] It crystallizes in the PuBr3 type[6][7] with space group Cmcm (No. 63) with a = 4.3281(6) Å, b = 14.003(6) Å and c = 9.988(3) Å.[8] It decomposes through an intermediate phase 2 PrI3·PrOI to a mixture of praseodymium oxyiodide and praseodymium oxide (5 PrOI·Pr2O3).[9]
Reactions
- PrI3 forms compounds with hydrazine, like I3Pr·3N2H4·4H2O which has pale yellow crystals and soluble in methanol, slightly soluble in water, and insoluble in benzene, d20 °C = 2.986 g/cm3.[10]
- PrI3 forms compounds with thiourea, like I3Pr·2CS(NH2)2·9H2O which is a green crystal with d = 2.27 g/cm3.[5][12]
- Praseodymium(III) iodide forms a nonahydrate, PrI3·9H2O. It can be obtained by dissolving praseodymium(III) oxide in concentrated aqueous hydroiodic acid:
- Pr2O3 + 6 HI + 15H2O → 2 PrI3·9H2O
- It adopts the same structure as other light rare earth iodides (La–Ho) and contains a triangular tricapped prismatic nonaaqua ion [Pr(OH2)9]3+ and iodide counterions.[4]
- Praseodymium(III) iodide reacts with praseodymium metal at elevated temperatures to form praseodymium diiodide:[13]
- 2 PrI3 + Pr → 3 PrI2
See also
References
- ↑ 1.0 1.1 1.2 1.3 Haynes, William M. (2016-06-22) (in en). CRC Handbook of Chemistry and Physics. CRC Press. pp. 2016–2652. ISBN 978-1-4987-5429-3. https://books.google.com/books?id=VVezDAAAQBAJ&pg=SA4-PA81%20CRC%20Handbook%20of%20Chemistry%20and%20Physics.
- ↑ Solubility_Table_Zh.PDF_version.pdf
- ↑ "Praseodymium triiodide" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/83744#section=Safety-and-Hazards.
- ↑ 4.0 4.1 Timofte, T.; Babai, A.; Meyer, G.; Mudring, A.-V. (2005). "Praseodymium triiodide nonahydrate". Acta Crystallogr. E 61 (6): i94–i95. doi:10.1107/S1600536805012857. Bibcode: 2005AcCrE..61I..94T.
- ↑ 5.0 5.1 5.2 "Solubility_Table_Zh". 27 August 2016. https://upload.wikimedia.org/wikipedia/commons/3/31/Solubility_Table_Zh.PDF_version.pdf.
- ↑ 6.0 6.1 Asprey, L. B.; Keenan, T. K.; Kruse, F. H. (1964). "Preparation and Crystal Data for Lanthanide and Actinide Triiodides". Inorg. Chem. 3 (8): 1137–1141. doi:10.1021/ic50018a015. https://digital.library.unt.edu/ark:/67531/metadc867868/.
- ↑ Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. p. 421. ISBN 978-0-19-965763-6.
- ↑ E. Warkentin, H. Bärnighausen (1979), "Die Kristallstruktur von Praseodymdiiodid (Modifikation V)" (in German), Zeitschrift für anorganische und allgemeine Chemie 459 (1): pp. 187–200, doi:10.1002/zaac.19794590120
- ↑ Heiniö, Outi; Leskelä, Markku; Niinistö, Lauri; Tuhtar, Dinko; Sjöblom, Johan; Strand, T. G.; Sukhoverkhov, V. F. (1980). "Structural and Thermal Properties of Rare Earth Triiodide Hydrates.". Acta Chemica Scandinavica 34a: 207–211. doi:10.3891/acta.chem.scand.34a-0207. ISSN 0904-213X.
- ↑ Uchenye zapiski: Serii︠a︡ khimicheskikh nauk (S.M. Kirov adyna Azărbai̐jan Dȯvlăt Universiteti; 1977), trang 37. Truy cập 1 tháng 1 năm 2021.
- ↑ Russian Journal of Inorganic Chemistry, Tập 18,Phần 2 (British Library Lending Division with the cooperation of the Royal Society of Chemistry, 1973), trang 1655. Truy cập 1 tháng 1 năm 2021.
- ↑ Villars, Pierre; Cenzual, Karin; Gladyshevskii, Roman (24 July 2017) (in en). Handbook of Inorganic Substances 2017. Walter de Gruyter GmbH & Co KG. p. 5090. ISBN 978-3-11-043655-6. https://books.google.com/books?id=ycw0DwAAQBAJ&pg=PT5090.
- ↑ Gerlitzki, Niels; Meyer, Gerd; Mudring, Anja-Verena; Corbett, John D. (2004). "Praseodymium diiodide, PrI2, revisited by synthesis, structure determination and theory". J. Alloys Compd. (Elsevier BV) 380 (1–2): 211–218. doi:10.1016/j.jallcom.2004.03.046. ISSN 0925-8388.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

