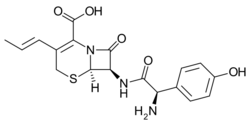
Chemistry:Cefprozil
 | |
| Clinical data | |
|---|---|
| Trade names | Cefzil, Cefproz, others |
| Other names | Cefproxil |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698022 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95% |
| Protein binding | 36% |
| Elimination half-life | 1.3 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H19N3O5S |
| Molar mass | 389.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cefprozil is a second-generation cephalosporin antibiotic.[1] Originally discovered in 1983, and approved in 1992,[2] it was sold under the tradename Cefzil by Bristol Meyers Squibb until 2010 when the brand name version was discontinued.[3] It continues to be available from various companies in its generic form.[4] It is used in the treatment of pharyngitis, tonsillitis, ear infections, acute sinusitis, bacterial exacerbation of chronic bronchitis, and skin and skin structure infections.[5] It is currently available as a tablet and as a liquid suspension.
Adverse effects
Although there is a widely quoted cross-allergy risk of 10% between cephalosporins and penicillin, research has shown no increased risk for cross-allergy for cefprozil and several other second-generation or later cephalosporins.[6] The most common side effects were increased hepatic lab values (including AST and ALGT), dizziness, eosinophilia, diaper rash and superinfection, genital pruritus, vaginitis, diarrhea, nausea, vomiting, and abdominal pain.[5]
Spectrum of bacterial susceptibility and resistance
Currently, bacteria like Enterobacter aerogenes, Morganella morganii and Pseudomonas aeruginosa are resistant to cefprozil, while Salmonella enterica serotype Agona and streptococci are susceptible to cefprozil. Some bacteria like Brucella abortus, Moraxella catarrhalis and Streptococcus pneumoniae have developed resistance towards cefprozil in varying degrees. Detailed minimum inhibition concentration information is given by the Cefprozil Susceptibility and Resistance Data sheet.[7]
Synthesis
This is not a direct copy of Lednicer book like at first glance, but is sourced from the primary reference material.

Displacement of the allylic chloride in intermediate (1) with triphenylphosphine gives the phosphonium salt (2). This functionality is then converted to its ylide; condensation with acetaldehyde then leads to the vinyl derivative (3); deprotection then gives cefprozil. Semisynthetic oral cephalosporin consisting of ~90:10 Z/E isomeric mixture.[12][13]
References
- ↑ "Cefzil (cefprozil) dosing, indications, interactions, adverse effects, and more". https://reference.medscape.com/drug/cefzil-cefprozil-342499.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 496. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA496.
- ↑ "Determination That CEFZIL (Cefprozil) Tablets, 250 Milligrams and 500 Milligrams, and for Oral Suspension, 125 Milligrams/5 Milliliters and 250 Milligrams/5 Milliliters, Were Not Withdrawn From Sale for Reasons of Safety or Effectiveness". National Archives. 11 September 2018. https://www.federalregister.gov/d/2018-19663.
- ↑ "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
- ↑ 5.0 5.1 "Cefzil® (CEFPROZIL) Prescribing Facts". Bristol Myers Squibb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050664s026,050665s026lbl.pdf.
- ↑ "Cephalosporins can be prescribed safely for penicillin-allergic patients". The Journal of Family Practice 55 (2): 106–112. February 2006. PMID 16451776. http://www.jfponline.com/pdf%2F5502%2F5502JFP%5FAppliedEvidence1%2Epdf. Retrieved 2011-02-26.
- ↑ "Cefprozil Susceptibility and Resistance Data". http://www.toku-e.com/Assets/MIC/Cefprozil.pdf.
- ↑ Hoshi H, et al., DE patent 3402642, issued 1984, assigned to Bristol-Myers
- ↑ Hoshi H, et al., US patent 4520022, issued 1985, assigned to Bristol-Myers
- ↑ "Synthesis and structure-activity relationships of a new oral cephalosporin, BMY-28100 and related compounds". The Journal of Antibiotics 40 (7): 991–1005. July 1987. doi:10.7164/antibiotics.40.991. PMID 3624077.
- ↑ Kaplan MA, et al., US patent 4727070, issued 1988, assigned to Bristol-Myers
- ↑ "In vitro and in vivo evaluations of BMY-28100, a new oral cephalosporin". The Journal of Antibiotics 40 (8): 1175–1183. August 1987. doi:10.7164/antibiotics.40.1175. PMID 3500158.
- ↑ "Forty days oral toxicity of 2,6-cis-diphenylhexamethylcyclotetrasiloxane (KABI 1774)in beagle dogs with special reference to effects on the male reproductive system". Acta Pharmacologica et Toxicologica 36 (Suppl 3): 93–130. 1975. doi:10.1111/j.1600-0773.1975.tb03087.x. PMID 1080338.
External links
- Cefprozil MedlinePlus Drug Information
 |
