Chemistry:Benzylpenicillin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Pfizerpen, other |
| Other names | Penicillin G potassium,[2] penicillin G sodium |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a685013 |
| Pregnancy category |
|
| Routes of administration | Intravenous therapy, intramuscular injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30% oral [3] |
| Protein binding | 60% |
| Metabolism | Liver |
| Elimination half-life | 30 min |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
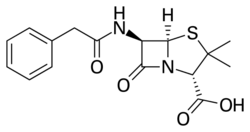
| Formula | C16H18N2O4S |
| Molar mass | 334.39 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benzylpenicillin, also known as penicillin G (PenG[4]) or BENPEN,[5] is an antibiotic used to treat a number of bacterial infections.[6] This includes pneumonia, strep throat, syphilis, necrotizing enterocolitis, diphtheria, gas gangrene, leptospirosis, cellulitis, and tetanus.[6] It is not a first-line agent for pneumococcal meningitis.[6] Due to benzylpenicillin's limited bioavailability for oral medications, it is generally taken as an injection in the form of a sodium, potassium, benzathine, or procaine salt.[7] Benzylpenicillin is given by injection into a vein or muscle.[2] Two long-acting forms benzathine benzylpenicillin and procaine benzylpenicillin are available for use by injection into a muscle only.[6]
Side effects include diarrhea, seizures, and allergic reactions including anaphylaxis.[6] When used to treat syphilis or Lyme disease a reaction known as Jarisch–Herxheimer may occur.[6] It is not recommended in those with a history of penicillin allergy.[6] Use during pregnancy is generally safe in the penicillin and β-lactam class of medications.[6]
Benzylpenicillin is on the World Health Organization's List of Essential Medicines.[8]
Medical uses
Antimicrobial potency
As an antibiotic, benzylpenicillin is noted to possess effectiveness mainly against gram-positive organisms. Some gram-negative organisms such as Neisseria gonorrhoeae and Leptospira weilii are also reported to be susceptible to benzylpenicillin.[9]
Adverse effects
Adverse effects can include hypersensitivity reactions including urticaria, fever, joint pains, rashes, angioedema, anaphylaxis, serum sickness-like reaction. Rarely central nervous system toxicity including convulsions (especially with high doses or in severe renal impairment), interstitial nephritis, haemolytic anaemia, leucopenia, thrombocytopenia, and coagulation disorders. Also reported diarrhoea (including antibiotic-associated colitis). Benzylpenicillin has relatively low toxicity, except for in the nervous system, in which it is one of the most active drugs among β-lactam agents.[7] In addition, benzylpenicillin is an irritant, a health hazard, and an environmental hazard.[10]
Benzylpenicillin serum concentrations can be monitored either by traditional microbiological assay or by more modern chromatographic techniques. Such measurements can be useful to avoid central nervous system toxicity in any person receiving large doses of the drug on a chronic basis, but they are especially relevant to patients with kidney failure, who may accumulate the drug due to reduced urinary excretion rates.[11][12]
Manufacture
Benzylpenicillin is produced by fermentation of Penicillium chrysogenum.[10] The production of benzylpenicillin involves fermentation, recovery and purification of the penicillin.[13]
The fermentation process of the production of benzylpenicillin creates the product. The presence of the product in solution inhibits the reaction and reduces the product rate and yield. Thus, in order to obtain the most product and increase the rate of reaction, it is continuously extracted.[14] This is done by mixing the mold with either glucose, sucrose, lactose, starch, or dextrin, nitrate, ammonium salt, corn steep liquor, peptone, meat or yeast extract, and small amounts of inorganic salts.[15]
The recovery of the benzylpenicillin is the most important part of the production process because it affects the later purification steps if done incorrectly.[13] There are several techniques used to recover benzylpenicillin: aqueous two-phase extraction, liquid membrane extraction, microfiltration, and solvent extraction.[13] Extraction is more commonly used in the recovery process.
In the purification step, the benzylpenicillin is separated from the extraction solution. This is normally done by using a separation column.[16]
Synonyms
References
- ↑ "Conformations of penicillin G: crystal structure of procaine penicillin G monohydrate and a refinement of the structure of potassium penicillin G". Journal of the Chemical Society, Perkin Transactions 1 3 (3): 185–190. 1978. doi:10.1039/p19780000185. PMID 565366.
- ↑ 2.0 2.1 "Penicillin G Injection - FDA prescribing information, side effects and uses". https://www.drugs.com/pro/penicillin-g-injection.html.
- ↑ Yip, Derek W.; Gerriets, Valerie (2023). Penicillin. StatPearls Publishing. PMID 32119447. https://www.ncbi.nlm.nih.gov/books/NBK554560. Retrieved 7 December 2023.
- ↑ "Immunogenicity and Antigenicity". Immunology for Pharmacy. Mosby. 2012. ISBN 978-0-323-06947-2. https://www.sciencedirect.com/book/9780323069472/immunology-for-pharmacy. "Natural penicillin (PenG), penicillinase-resistant penicillin (methicillin), extended-spectrum penicillin (amoxicillin), and broad-spectrum penicillin (carbenicillin) all have the same core β-lactam ring, which is essential for antimicrobial activity."
- ↑ "Australian Product Information – BENPEN". Seqirus Pty Ltd. https://labeling.seqirus.com/PI/AU/Benpen/EN/Benpen-Product-Information.pdf.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 WHO Model Formulary 2008. World Health Organization. 2009. pp. 98, 105. ISBN 9789241547659.
- ↑ 7.0 7.1 "Penicillin G". xPharm: The Comprehensive Pharmacology Reference. New York: Elsevier. 2007. pp. 1–6.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Penicillin G". Toku-E. 2010-10-10. http://www.toku-e.com/Upload/Products/PDS/20120521008049.pdf.
- ↑ 10.0 10.1 10.2 "Benzylpenicillin". Molecule of the Week. American Chemical Society. https://www.acs.org/content/acs/en/molecule-of-the-week/archive/b/benzylpenicillin.html.
- ↑ "Neurotoxicity during intravenous infusion of penicillin. A review". Journal of Clinical Pharmacology 14 (10): 504–12. October 1974. doi:10.1002/j.1552-4604.1974.tb01364.x. PMID 4610013.
- ↑ Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. 2008. pp. 1195–1196.
- ↑ 13.0 13.1 13.2 "Efficient Recovery of Penicillin G by a Hydrophobic Ionic Liquid". ACS Sustainable Chemistry & Engineering 4 (2): 609–615. February 2016. doi:10.1021/acssuschemeng.5b00975.
- ↑ "Use Extraction to Improve Penicillin G Recovery". Discover Chemistry. American Chemical Society. 4 January 2016. https://www.acs.org/content/acs/en/pressroom/cutting-edge-chemistry/use-extraction-to-improve-penicillin-g-recovery.html.
- ↑ "Separation and Purification of Pharmaceuticals and Antibiotics". Mitsubishi Chemical Corporation. pp. 312–324. https://www.diaion.com/en/application/pharmaceutical/pdf/antibiotics_fermentation_products_small_molecules_apis.pdf.
- ↑ "Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia". Antimicrobial Agents and Chemotherapy 22 (4): 564–570. October 1982. doi:10.1128/AAC.22.4.564. PMID 6983856.
- ↑ "Chemistry of penicillin". The Analyst 72 (856): 274–276. July 1947. doi:10.1039/an9477200274. PMID 20259048. Bibcode: 1947Ana....72..274R.
- ↑ "Penicillin G". National Center for Biotechnology Information, National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/Penicillin-g.
 |

