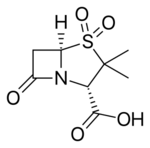
Chemistry:Sulbactam
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a693021 |
| Routes of administration | Intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 29% |
| Elimination half-life | 0.65–1.20 hrs |
| Excretion | Mainly kidneys (41–66% within 8 hrs) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C8H11NO5S |
| Molar mass | 233.24 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 148 to 151 °C (298 to 304 °F) |
| |
| |
| (verify) | |
Sulbactam is a β-lactamase inhibitor. This drug is given in combination with β-lactam antibiotics to inhibit β-lactamase, an enzyme produced by bacteria that destroys the antibiotics.[1]
It was patented in 1977 and approved for medical use in 1986.[2]
Medical uses
The combination ampicillin/sulbactam (Unasyn) is available in the United States.[3]
The combination cefoperazone/sulbactam (Sulperazon) is available in many countries.[4]
The co-packaged combination sulbactam/durlobactam was approved for medical use in the United States in May 2023.[5]
Mechanism
Sulbactam is primarily used as a suicide inhibitor of β-lactamase, shielding more potent beta-lactams such as ampicillin.[6] Sulbactam itself contains a beta-lactam ring, and has weak antibacterial activity by inhibiting penicillin binding proteins (PBP) 1 and 3, but not 2.[7]
References
- ↑ "Sulbactam forms only minimal amounts of irreversible acrylate-enzyme with SHV-1 beta-lactamase". Biochemistry 46 (31): 8980–8987. August 2007. doi:10.1021/bi7006146. PMID 17630699.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 492. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA492.
- ↑ "Unasyn- ampicillin sodium and sulbactam sodium injection, powder, for solution". DailyMed. U.S. National Library of Medicine. 29 March 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12eeb72a-403d-41be-bae4-4cb930862884.
- ↑ "Sulperazon". drugs.com. https://www.drugs.com/international/sulperazon.html.
- ↑ "FDA Approves New Treatment for Pneumonia Caused by Certain Difficult-to-Treat Bacteria". U.S. Food and Drug Administration (Press release). 24 May 2023. Retrieved 24 May 2023.
- ↑ "Pharmacokinetics and Pharmacodynamics of β-Lactamase Inhibitors". Pharmacotherapy 39 (2): 182–195. February 2019. doi:10.1002/phar.2210. PMID 30589457.
- ↑ "Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii". Antimicrobial Agents and Chemotherapy 59 (3): 1680–1689. March 2015. doi:10.1128/AAC.04808-14. PMID 25561334.
Further reading
- "Beta-lactams in the new millennium. Part-II: cephems, oxacephems, penams and sulbactam". Mini Reviews in Medicinal Chemistry 4 (1): 93–109. January 2004. doi:10.2174/1389557043487547. PMID 14754446.
 |
