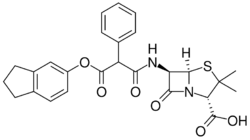
Chemistry:Carindacillin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H26N2O6S |
| Molar mass | 494.56 g·mol−1 |
| 3D model (JSmol) | |
| |
Carindacillin (INN), also known as carbenicillin indanyl (USAN), is a penicillin antibiotic. It is a prodrug of carbenicillin.[1]
It is administered orally, as the sodium salt. It was formerly marketed in the United States by Pfizer under the brand name Geocillin. Pfizer withdrew Carindacillin from the U.S. market sometime after 2008.[2]
Pharmacokinetics
Shortly after absorption via the small intestine, Carindacillin is hydrolyzed into Carbenicillin. Carbenicillin acts by interfering with final cell wall synthesis in susceptible bacteria, including Pseudomonas aeruginosa, Escherichia coli, and some Proteus. The most common adverse effects include nausea, bad taste, diarrhea, vomiting, flatulence, and glossitis. Carindacillin was approved for use in adults in the treatment of prostatitis and urinary tract infections. Dosing was between 2000 and 4000 mg daily, divided into equally spaced doses.[3]
References
- ↑ "Carbenicillin indanyl sodium, an orally active derivative of carbenicillin". Antimicrob. Agents Chemother. 1 (3): 185–91. March 1972. doi:10.1128/aac.1.3.185. PMID 4558137.
- ↑ "Drugs@FDA: FDA-Approved Drugs CARBENICILLIN INDANYL SODIUM". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=050435.
- ↑ Pfizer (2008). "GEOCILLIN® carbenicillin indanyl sodium tablets label". https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050435s009lbl.pdf.
 |

