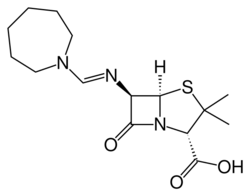
Chemistry:Mecillinam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Coactin, Leo, Selexid, Selexidin |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Negligible |
| Protein binding | 5 to 10% |
| Metabolism | Some hepatic metabolism |
| Elimination half-life | 1 to 3 hours |
| Excretion | Renal and biliary, mostly unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C15H23N3O3S |
| Molar mass | 325.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| | |
Mecillinam (INN) or amdinocillin (USAN) is an extended-spectrum penicillin antibiotic of the amidinopenicillin class that binds specifically to penicillin binding protein 2 (PBP2),[2] and is only considered to be active against Gram-negative bacteria. It is used primarily in the treatment of urinary tract infections, and has also been used to treat typhoid and paratyphoid fever.[3][4] Because mecillinam has very low oral bioavailability, an orally active prodrug was developed: pivmecillinam.
Medical uses
Mecillinam is used in the treatment of infections due to susceptible gram-negative bacteria, especially urinary tract infections which are most commonly caused by Escherichia coli.[5] Mecillinam is active against most pathogenic Gram-negative bacteria, except Pseudomonas aeruginosa and some species of Proteus.[6] Several studies have also found it to be as effective as other antibiotics for treating Staphylococcus saprophyticus infection, though it is Gram-positive, possibly because mecillinam reaches very high concentrations in urine.[1]
Worldwide resistance to mecillinam in bacteria causing urinary tract infection has remained very low since its introduction; a 2003 study conducted in 16 European countries and Canada found resistance to range from 1.2% (Escherichia coli) to 5.2% (Proteus mirabilis).[7] Another large study conducted in Europe and Brazil obtained similar results — 95.9% of E. coli strains, for instance, were sensitive to mecillinam.[8]
Adverse effects
The adverse effect profile of mecillinam is similar to that of other penicillins.[2] Its most common side effects are rash and gastrointestinal upset, including nausea and vomiting.[1]
History
With the codename FL 1060, mecillinam was developed by the Danish pharmaceutical company Leo Pharmaceutical Products (now LEO Pharma). It was first described in the scientific literature in a 1972 paper.[9][10]
References
- ↑ 1.0 1.1 1.2 "Pivmecillinam in the treatment of urinary tract infections". The Journal of Antimicrobial Chemotherapy 46 (Suppl A): 35–39. August 2000. doi:10.1093/jac/46.suppl_1.35. PMID 10969050.
- ↑ 2.0 2.1 "Amdinocillin: a novel penicillin. Antibacterial activity, pharmacology and clinical use". Pharmacotherapy 5 (1): 1–10. 1985. doi:10.1002/j.1875-9114.1985.tb04448.x. PMID 3885172.
- ↑ "Mecillinam: a new antibiotic for enteric fever". British Medical Journal 2 (6026): 14–15. July 1976. doi:10.1136/bmj.2.6026.14. PMID 820402.
- ↑ "The treatment of enteric fever with mecillinam". The Journal of Antimicrobial Chemotherapy 3 (Suppl B): 101–102. July 1977. doi:10.1093/jac/3.suppl_b.101. PMID 408321.
- ↑ "[National S3 guideline on uncomplicated urinary tract infection: recommendations for treatment and management of uncomplicated community-acquired bacterial urinary tract infections in adult patients]" (in de). Der Urologe. Ausg. A 50 (2): 153–169. February 2011. doi:10.1007/s00120-011-2512-z. PMID 21312083.
- ↑ "Amdinocillin (Mecillinam)". Point-of-Care Information Technology ABX Guide. Johns Hopkins University. August 28, 2008. http://prod.hopkins-abxguide.org/antibiotics/antibacterial/pcn_others/amdinocillin__mecillinam_.html. Retrieved on August 31, 2008. Freely available with registration.
- ↑ "An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project". The Journal of Antimicrobial Chemotherapy 51 (1): 69–76. January 2003. doi:10.1093/jac/dkg028. PMID 12493789.
- ↑ "Surveillance study in Europe and Brazil on clinical aspects and Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC): implications for empiric therapy". European Urology 54 (5): 1164–1175. November 2008. doi:10.1016/j.eururo.2008.05.010. PMID 18511178.
- ↑ "6 -amidinopenicillanic acids--a new group of antibiotics". Nature 236 (66): 135–137. April 1972. doi:10.1038/236135c0. PMID 4402006.
- ↑ "Mecillinam (FL 1060), a 6beta-amidinopenicillanic acid derivative: bactericidal action and synergy in vitro". Antimicrobial Agents and Chemotherapy 8 (3): 271–276. September 1975. doi:10.1128/aac.8.3.271. PMID 170856.
 |
