Chemistry:Temocillin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
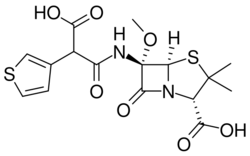
| Formula | C16H18N2O7S2 |
| Molar mass | 414.45 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Temocillin is a β-lactamase-resistant penicillin[1][2] introduced by Beecham, marketed by Eumedica Pharmaceuticals as Negaban. It is used primarily for the treatment of multiple drug-resistant, Gram-negative bacteria.
It is a 6-methoxy penicillin; it is also a carboxypenicillin.[3]
Pharmacology
Temocillin is a β-lactamase-resistant penicillin. It is not active against Gram-positive bacteria or bacteria with altered penicillin-binding proteins.[citation needed]
It is normally active against Moraxella catarrhalis, Brucella abortus, Burkholderia cepacia, Citrobacter species, Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Pasteurella multocida, Proteus mirabilis, Salmonella typhimurium, and Yersinia enterocolitica. It is also active against some Enterobacter species, Morganella morganii, and Serratia species. Temocillin has no useful activity against Acinetobacter species or Pseudomonas aeruginosa.[4]
Its primary use is against Enterobacteriaceae, and in particular against strains producing extended-spectrum β-lactamase or AmpC β-lactamase.[5]
Dosage
The common dose is 2 g intravenously every 12 hours and the high dose, notably in critically ill patients, is 2g every 8 hours. Theoretical reasons exist for giving temocillin as a continuous intravenous infusion in severe disease[6][7] a single loading dose of 2 g is given intravenously followed by a 4-g or 6-g infusion over 24 hours. According to the SPC, chemical and physical in-use stability has been demonstrated for 24 hours at 25 °C for the following solvents: water for injection, physiological saline (0.9% sodium chloride), dextrose 5%, sodium chloride compound (Ringer's solution), Hartmann solution (sodium lactate compound + Ringer's lactate solution). Temocillin for intravenous injection is diluted in 10 to 20 ml of sterile water; it is diluted in less than 2 ml of sterile water when being prepared for intramuscular injection; the continuous infusion is diluted in 48 ml of sterile water for ease of administration (2 ml per hour). To reduce pain, the intramuscular injection may be made up using sterile 1% lignocaine instead of sterile water.[citation needed]
Temocillin may be given to patients with impaired renal function after the dose has been adapted:
| Creatinine clearance (mL/min) | Dosage per administration | Interval between administrations |
|---|---|---|
| More than 60 | 2 g | 12 h |
| 60 to 30 | 1 g | 12 h |
| 30 to 10 | 1 g | 24 h |
In case of intermittent high-flux hemodialysis: 1 g (I.V. injection) per 24 h of inter-dialytic session, preferably at the end of the hemodialysis (1 g q24 h, 2 g q48 h, 3 g q72 h). In case of continuous peritoneal dialysis in ambulatory patients: 1 g every 24 hours.
No oral preparation of temocillin is licensed.
Adverse effects
The undesirable effects of temocillin are those of any β-lactam antibiotic. In particular, it has been associated with angioedema and anaphylaxis in penicillin-allergic patients. Animal studies have not shown any induction of Clostridium difficile infection.[8] As with any other penicillin, convulsions can occur if very high doses are given.[citation needed]
Synthesis

References
- ↑ "Temocillin susceptibility by BSAC methodology". The Journal of Antimicrobial Chemotherapy 60 (1): 185–187. July 2007. doi:10.1093/jac/dkm179. PMID 17550891.
- ↑ "In vitro activity of temocillin (BRL 17421), a novel beta-lactam antibiotic". Antimicrobial Agents and Chemotherapy 22 (4): 535–540. October 1982. doi:10.1128/aac.22.4.535. PMID 7181470.
- ↑ "[In vitro study of the bacteriostatic and bactericidal activity of temocillin (BRL 17421)]" (in fr). Pathologie-Biologie 31 (6): 467–470. June 1983. PMID 6348653.
- ↑ "Temocillin: Applications in Antimicrobial Stewardship as a Potential Carbapenem-Sparing Antibiotic". Antibiotics 11 (4): 493. April 2022. doi:10.3390/antibiotics11040493. PMID 35453244.
- ↑ "Activity of temocillin against prevalent ESBL- and AmpC-producing Enterobacteriaceae from south-east England". The Journal of Antimicrobial Chemotherapy 57 (5): 1012–4. May 2006. doi:10.1093/jac/dkl043. PMID 16531428.
- ↑ "Continuous versus intermittent infusion of temocillin, a directed spectrum penicillin for intensive care patients with nosocomial pneumonia: stability, compatibility, population pharmacokinetic studies and breakpoint selection". The Journal of Antimicrobial Chemotherapy 61 (2): 382–8. February 2008. doi:10.1093/jac/dkm467. PMID 18070831.
- ↑ "Temocillin (6 g daily) in critically ill patients: continuous infusion versus three times daily administration". The Journal of Antimicrobial Chemotherapy 70 (3): 891–8. March 2015. doi:10.1093/jac/dku465. PMID 25433006.
- ↑ "Studies with temocillin in a hamster model of antibiotic-associated colitis". Antimicrobial Agents and Chemotherapy 27 (6): 980–981. June 1985. doi:10.1128/aac.27.6.980. PMID 3875312.
- ↑ "Transformations using benzyl 6-isocyanopenicillanate". Journal of the Chemical Society, Perkin Transactions 1: 2455. 1979. doi:10.1039/P19790002455.
Further reading
- "Temocillin revived". The Journal of Antimicrobial Chemotherapy 63 (2): 243–245. February 2009. doi:10.1093/jac/dkn511. PMID 19095679.
 |
