Chemistry:Dehydronorketamine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
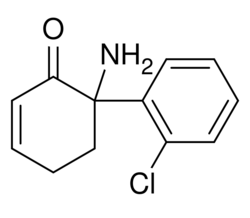
| Formula | C12H12ClNO |
| Molar mass | 221.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dehydronorketamine (DHNK), or 5,6-dehydronorketamine, is a minor metabolite of ketamine which is formed by dehydrogenation of its metabolite norketamine.[1][2] Though originally considered to be inactive,[1][2][3] DHNK has been found to act as a potent and selective negative allosteric modulator of the α7-nicotinic acetylcholine receptor (IC50 = 55 nM).[4][5] For this reason, similarly to hydroxynorketamine (HNK), it has been hypothesized that DHNK may have the capacity to produce rapid antidepressant effects.[6] However, unlike ketamine, norketamine, and HNK, DHNK has been found to be inactive in the forced swim test (FST) in mice at doses up to 50 mg/kg.[7] DHNK is inactive at the α3β4-nicotinic acetylcholine receptor (IC50 > 100 μM) and is only very weakly active at the NMDA receptor (Ki = 38.95 μM for (S)-(+)-DHNK).[4] It can be detected 7–10 days after a modest dose of ketamine, and because of this, is useful in drug detection assays.[8]
See also
References
- ↑ 1.0 1.1 "Intravenous Agents". Pediatric Anesthesia. PMPH-USA. 14 May 2014. pp. 366–. ISBN 978-1-60795-213-8. https://books.google.com/books?id=yq8tAwAAQBAJ&pg=PA366.
- ↑ 2.0 2.1 "Ketamine in treatment-resistant depression". Clinical Handbook for the Management of Mood Disorders. Cambridge University Press. 9 May 2013. pp. 345–357 (347). doi:10.1017/CBO9781139175869.027. ISBN 978-1-107-06744-8. https://books.google.com/books?id=3NPvLExs7X8C&pg=PA347.
- ↑ ""Addicted to Euphoria": The History, Clinical Presentation, and Management of Party Drug Misuse". International Review of Neurobiology (Elsevier Science) 120: 205–233 (225). 2015. doi:10.1016/bs.irn.2015.02.005. ISBN 978-0-12-803003-5. PMID 26070759. https://books.google.com/books?id=qUSnBQAAQBAJ&pg=PA225.
- ↑ 4.0 4.1 "Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors". European Journal of Pharmacology 698 (1–3): 228–234. January 2013. doi:10.1016/j.ejphar.2012.11.023. PMID 23183107.
- ↑ Nicotinic Receptors. Springer. 11 November 2014. pp. 445–. ISBN 978-1-4939-1167-7. https://books.google.com/books?id=Y0BTBQAAQBAJ&pg=PA445.
- ↑ "(R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function". Anesthesiology 121 (1): 149–159. July 2014. doi:10.1097/ALN.0000000000000285. PMID 24936922.
- ↑ "Antidepressant-like effects of ketamine, norketamine and dehydronorketamine in forced swim test: Role of activity at NMDA receptor". Neuropharmacology 99: 301–307. December 2015. doi:10.1016/j.neuropharm.2015.07.037. PMID 26240948.
- ↑ Ultra-High Performance Liquid Chromatography and Its Applications. John Wiley & Sons. 1 April 2013. pp. 1–. ISBN 978-1-118-53398-7. https://books.google.com/books?id=pl80RKCNkQ0C&pg=SA1-PA145.
 |

