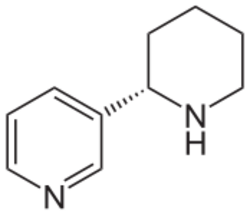
Chemistry:Anabasine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C10H14N2 |
| Molar mass | 162.236 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Anabasine is a pyridine and piperidine alkaloid found in the Tree Tobacco (Nicotiana glauca) plant, as well as in the close relative of the common tobacco plant (Nicotiana tabacum).[1] It is a structural isomer of, and chemically similar to, nicotine. Its principal (historical) industrial use is as an insecticide.
Anabasine is present in trace amounts in tobacco smoke, and can be used as an indicator of a person's exposure to tobacco smoke.[2]
Pharmacology
Anabasine is a nicotinic acetylcholine receptor agonist. In high doses, it produces a depolarizing block of nerve transmission, which can cause symptoms similar to those of nicotine poisoning and, ultimately, death by asystole.[3] In larger amounts it is thought to be teratogenic in swine.[4]
The intravenous LD50 of anabasine ranges from 11 mg/kg to 16 mg/kg in mice, depending on the enantiomer.[5]
Analogs
B. Bhatti, et al. made some higher potency sterically strained bicyclic analogs of anabasine:[6]
- 2-(Pyridin-3-yl)-1-azabicyclo[3.2.2]nonane (TC-1698)
- 2-(Pyridin-3-yl)-1-azabicyclo[2.2.2]octane,
- and 2-(Pyridin-3-yl)-1-azabicyclo[3.2.1]octane.
See also
References
- ↑ "Fractionation and Extraction Optimization of Potentially Valuable Compounds and Their Profiling in Six Varieties of Two Nicotiana Species". Molecules 27 (22): 8105. November 2022. doi:10.3390/molecules27228105. PMID 36432206.
- ↑ "Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes". American Journal of Public Health 89 (5): 731–6. May 1999. doi:10.2105/AJPH.89.5.731. PMID 10224986.
- ↑ "Fatal poisoning from Nicotiana glauca leaves: identification of anabasine by gas-chromatography/mass spectrometry". Journal of Forensic Sciences 45 (3): 736–41. May 2000. doi:10.1520/JFS14761J. PMID 10855991.
- ↑ "Notes on Poisoning: Nicotiana tabacum". Canadian Biodiversity Information Facility. 2008-03-18. http://www.cbif.gc.ca/pls/pp/ppack.info?p_psn=186&p_type=all&p_sci=sci&p_x=px.
- ↑ "Relative toxicities and neuromuscular nicotinic receptor agonistic potencies of anabasine enantiomers and anabaseine". Neurotoxicology and Teratology 28 (2): 220–8. 2006. doi:10.1016/j.ntt.2005.12.010. PMID 16488116. https://naldc-legacy.nal.usda.gov/naldc/download.xhtml?id=21887&content=PDF.
- ↑ "Synthesis of 2-(pyridin-3-yl)-1-azabicyclo[3.2.2]nonane, 2-(pyridin-3-yl)-1-azabicyclo[2.2.2]octane, and 2-(pyridin-3-yl)-1-azabicyclo[3.2.1]octane, a class of potent nicotinic acetylcholine receptor-ligands". The Journal of Organic Chemistry 73 (9): 3497–507. May 2008. doi:10.1021/jo800028q. PMID 18363376.
 |
