Chemistry:ABT-418
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
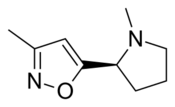
| Formula | C9H14N2O |
| Molar mass | 166.22 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
ABT-418 is a drug developed by Abbott, that has nootropic, neuroprotective and anxiolytic effects,[1][2][3][4][5] and has been researched for treatment of both Alzheimer's disease[6] and ADHD.[7][8][9] It acts as an agonist at neural nicotinic acetylcholine receptors, subtype-selective binding with high affinity to the α4β2, α7/5-HT3, and α2β2 nicotinic acetylcholine receptors but not α3β4 receptors[10][11][12] ABT-418 was reasonably effective for both applications and fairly well tolerated, but produced some side effects, principally nausea, and it is unclear whether ABT-418 itself will proceed to clinical development or if another similar drug will be used instead.[13]
See also
References
- ↑ "(S)-3-methyl-5-(1-methyl-2-pyrrolidinyl) isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities: I. In vitro characterization". The Journal of Pharmacology and Experimental Therapeutics 270 (1): 310–318. July 1994. PMID 7518514. http://jpet.aspetjournals.org/cgi/content/abstract/270/1/310.
- ↑ "(S)-3-methyl-5-(1-methyl-2-pyrrolidinyl)isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities: II. In vivo characterization". The Journal of Pharmacology and Experimental Therapeutics 270 (1): 319–328. July 1994. PMID 7913497. http://jpet.aspetjournals.org/cgi/content/abstract/270/1/319.
- ↑ "Anxiolytic-like effects of the novel cholinergic channel activator ABT-418". The Journal of Pharmacology and Experimental Therapeutics 271 (1): 353–361. October 1994. PMID 7965735. http://jpet.aspetjournals.org/cgi/reprint/271/1/353.
- ↑ "Improvement in accuracy of delayed recall in aged and non-aged, mature monkeys after intramuscular or transdermal administration of the CNS nicotinic receptor agonist ABT-418". Psychopharmacology 130 (3): 276–284. April 1997. doi:10.1007/s002130050240. PMID 9151363.
- ↑ "Synthesis of novel chiral Δ2-isoxazoline derivatives related to ABT-418 and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes". European Journal of Medicinal Chemistry 45 (12): 5594–5601. December 2010. doi:10.1016/j.ejmech.2010.09.009. PMID 20932609.
- ↑ "Acute effects of the selective cholinergic channel activator (nicotinic agonist) ABT-418 in Alzheimer's disease". Psychopharmacology 142 (4): 334–342. March 1999. doi:10.1007/s002130050897. PMID 10229057.
- ↑ "A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder". The American Journal of Psychiatry 156 (12): 1931–1937. December 1999. doi:10.1176/ajp.156.12.1931. PMID 10588407.
- ↑ "Present and future pharmacotherapeutic options for adult attention deficit/hyperactivity disorder". Expert Opinion on Pharmacotherapy 2 (4): 573–586. April 2001. doi:10.1517/14656566.2.4.573. PMID 11336608.
- ↑ "Non-stimulant treatment for Attention-Deficit/Hyperactivity Disorder". Journal of Attention Disorders 6 (Suppl 1): S109–S119. 2002. doi:10.1177/070674370200601s13. PMID 12685525.
- ↑ "Activation and inhibition of rat neuronal nicotinic receptors by ABT-418". British Journal of Pharmacology 120 (3): 429–438. February 1997. doi:10.1038/sj.bjp.0700930. PMID 9031746.
- ↑ "Synthesis of novel chiral Δ2-isoxazoline derivatives related to ABT-418 and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes". European Journal of Medicinal Chemistry 45 (12): 5594–5601. December 2010. doi:10.1016/j.ejmech.2010.09.009. PMID 20932609.
- ↑ "Human alpha 7 nicotinic acetylcholine receptor responses to novel ligands". Neuropharmacology 34 (6): 583–590. June 1995. doi:10.1016/0028-3908(95)00028-5. PMID 7566493.
- ↑ "Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition". Biochemical Pharmacology 74 (8): 1212–1223. October 2007. doi:10.1016/j.bcp.2007.07.002. PMID 17689498.
 |
