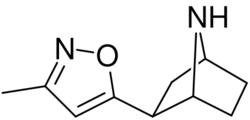
Chemistry:Epiboxidine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C10H14N2O |
| Molar mass | 178.235 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Epiboxidine is a chemical compound which acts as a partial agonist at neural nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes. It was developed as a less toxic analogue of the potent frog-derived alkaloid epibatidine, which is around 200 times stronger than morphine as an analgesic but produces extremely dangerous toxic nicotinic side effects.
Epiboxidine is around one-tenth as potent as epibatidine as an α4β2 agonist, but has around the same potency as an α3β4 agonist. It has only one-tenth of the analgesic power of epibatidine, but is also much less toxic.[1][2][3]
Uses
Despite its decreased potency and toxicity compared to epibatidine, epiboxidine itself is still too toxic to be developed as a drug for use in humans. It is used in scientific research[4] and as a parent compound to derive newer analogues which may be safer and have greater potential for clinical development.[5][6][7]
See also
References
- ↑ "Epiboxidine and novel-related analogues: a convenient synthetic approach and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes". Bioorganic & Medicinal Chemistry Letters 18 (16): 4651–4. August 2008. doi:10.1016/j.bmcl.2008.07.016. PMID 18644719. https://air.unimi.it/bitstream/2434/59291/1/Bioorganic%20%26%20Medicinal%20Chemistry%20Letters%2018%20%282008%29%204651%e2%80%934654.pdf.
- ↑ "The enantiomers of epiboxidine and of two related analogs: synthesis and estimation of their binding affinity at α4β2 and α7 neuronal nicotinic acetylcholine receptors". Chirality 24 (7): 543–51. July 2012. doi:10.1002/chir.22052. PMID 22566097.
- ↑ "Synthesis and nicotinic activity of epiboxidine: an isoxazole analogue of epibatidine". European Journal of Pharmacology 321 (2): 189–94. February 1997. doi:10.1016/S0014-2999(96)00939-9. PMID 9063687. https://zenodo.org/record/1259579.
- ↑ "Nicotine exposure refines visual map topography through an NMDA receptor-mediated pathway". The European Journal of Neuroscience 24 (11): 3026–42. December 2006. doi:10.1111/j.1460-9568.2006.05204.x. PMID 17156364.
- ↑ "Homoepiboxidines: further potent agonists for nicotinic receptors". Bioorganic & Medicinal Chemistry 12 (1): 179–90. January 2004. doi:10.1016/j.bmc.2003.10.015. PMID 14697783.
- ↑ "Synthesis and nicotinic acetylcholine receptor binding affinities of 2- and 3-isoxazolyl-8-azabicyclo[3.2.1]octanes". Bioorganic & Medicinal Chemistry Letters 14 (7): 1775–8. April 2004. doi:10.1016/j.bmcl.2004.01.025. PMID 15026069.
- ↑ "Aza-Prins-pinacol approach to 7-azabicyclo[2.2.1]heptanes: syntheses of (+/-)-epibatidine and (+/-)-epiboxidine". The Journal of Organic Chemistry 72 (21): 8019–24. October 2007. doi:10.1021/jo701536a. PMID 17867705.
 |
