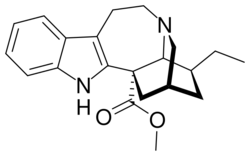
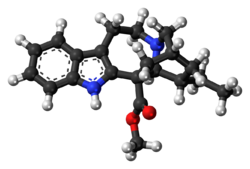
Chemistry:Coronaridine
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C21H26N2O2 |
| Molar mass | 338.451 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Coronaridine, also known as 18-carbomethoxyibogamine, is an alkaloid found in Tabernanthe iboga and related species, including Tabernaemontana divaricata for which (under the now obsolete synonym Ervatamia coronaria) it was named.[1]
Like ibogaine, (R)-coronaridine and (S)-coronaridine can decrease intake of cocaine and morphine in animals[2] and it may have muscle relaxant and hypotensive activity.[3]
Chemistry
Congeners
Coronaridine congers are important in drug discovery and development due to multiple actions on different targets. They have ability to inhibit Cav2.2 channel,[4] modulate and inhibit subunits of nAChr selectively such as α9α10,[4] α3β4[5][6] and potentiate GABAA activity.[7]
Pharmacology
Coronaridine has been reported to bind to an assortment of molecular sites, including: μ-opioid (Ki = 2.0 μM), δ-opioid (Ki = 8.1 μM), and κ-opioid receptors (Ki = 4.3 μM), NMDA receptor (Ki = 6.24 μM) (as an antagonist),[8] and nAChRs (as an antagonist).[9] It has also been found to inhibit the enzyme acetylcholinesterase, act as a voltage-gated sodium channel blocker,[10] and displays estrogenic activity in rodents.[8][9] In contrast to ibogaine and other iboga alkaloids, coronaridine does not bind to either the σ1 or σ2 receptor.[10]
Sources
| Family | Plants |
|---|---|
| Apocynaceae | T. catharinensis, T. ternifolia, T. pandacaqui, T. heyneana, T. litoralis, T. divaricata, T. penduliflora.[11] |
See also
References
- ↑ "In vitro activities of iboga alkaloid congeners coronaridine and 18-methoxycoronaridine against Leishmania amazonensis". Antimicrobial Agents and Chemotherapy 46 (7): 2111–2115. July 2002. doi:10.1128/aac.46.7.2111-2115.2002. PMID 12069962.
- ↑ The Psychopharmacology of Herbal Medicine: Plant Drugs that Alter Mind, Brain, and Behavior. The MIT Press; Illustrated edition. 2001. ISBN 978-0262692656. https://books.google.com/books?id=jZeaRiIFbhsC&q=desethylcoronaridine&pg=PA370.
- ↑ "Muscle relaxant activity and hypotensive activity of some Tabernaemontana alkaloids". Journal of Ethnopharmacology 13 (2): 165–173. May 1985. doi:10.1016/0378-8741(85)90004-2. PMID 4021514.
- ↑ 4.0 4.1 "Coronaridine congeners decrease neuropathic pain in mice and inhibit α9α10 nicotinic acetylcholine receptors and CaV2.2 channels". Neuropharmacology 175: 108194. September 2020. doi:10.1016/j.neuropharm.2020.108194. PMID 32540451.
- ↑ "Coronaridine congeners inhibit human α3β4 nicotinic acetylcholine receptors by interacting with luminal and non-luminal sites". The International Journal of Biochemistry & Cell Biology 65: 81–90. August 2015. doi:10.1016/j.biocel.2015.05.015. PMID 26022277.
- ↑ "Coronaridine congeners modulate mitochondrial α3β4* nicotinic acetylcholine receptors with different potency and through distinct intra-mitochondrial pathways". Neurochemistry International 114: 26–32. March 2018. doi:10.1016/j.neuint.2017.12.008. PMID 29277577.
- ↑ "Coronaridine congeners potentiate GABAA receptors and induce sedative activity in mice in a benzodiazepine-insensitive manner". Progress in Neuro-psychopharmacology & Biological Psychiatry 101: 109930. July 2020. doi:10.1016/j.pnpbp.2020.109930. PMID 32194202. https://hal.archives-ouvertes.fr/hal-03489760/file/S027858461931019X.pdf.
- ↑ 8.0 8.1 Lead Compounds from Medicinal Plants for the Treatment of Neurodegenerative Diseases. Academic Press. 16 December 2013. pp. 67–69, 73. ISBN 978-0-12-398383-1. https://books.google.com/books?id=o3opAgAAQBAJ&pg=PA67.
- ↑ 9.0 9.1 Biochemical Targets of Plant Bioactive Compounds: A Pharmacological Reference Guide to Sites of Action and Biological Effects. CRC Press. 15 May 2003. pp. 203–. ISBN 978-0-203-01371-7. https://books.google.com/books?id=Q20_AJ3wQOoC&pg=PA203.
- ↑ 10.0 10.1 "Pharmacology of Ibogaine and Ibogaine-Related Alkaloids". The Alkaloids. Chemistry and Biology. 52. San Diego: Academic Press. 1999. pp. 197–232 (222). ISBN 978-0-08-086576-8. https://books.google.com/books?id=bE503LRsawYC&pg=PA22.
- ↑ "(−)-Coronaridine". ChEBI. European Bioinformatics Institute. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:3887.
Treatment of drug dependence (N07B) | |
|---|---|
| Nicotine dependence | |
| Alcohol dependence | |
| Opioid dependence | |
| Benzodiazepine dependence | |
| Research | |
 | 0.00      (0 votes) (0 votes) |
