Chemistry:AR-R17779
From HandWiki
| |
| |
| Names | |
|---|---|
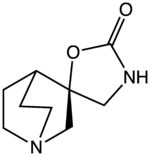
| IUPAC name
(2S)-4′-Azaspiro[bicyclo[2.2.2]octane-2,5′-[1,3]oxazolidin]-2′-one
| |
| Other names
(−)-Spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidin-2′-one]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H14N2O2 | |
| Molar mass | 182.223 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
AR-R17779 is a drug that acts as a potent and selective full agonist for the α7 subtype of neural nicotinic acetylcholine receptors.[1][2] It has nootropic effects in animal studies,[3][4] but its effects do not substitute for those of nicotine.[5] It has also been studied as a potential novel treatment for arthritis.[6]
References
- ↑ Mullen, G.; Napier, A.; Balestra, M.; Decory, T.; Hale, G.; Macor, J.; Mack, R.; Loch, J. et al. (2000). "(−)-Spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidin-2′-one], a conformationally restricted analogue of acetylcholine, is a highly selective full agonist at the α7 nicotinic acetylcholine receptor". Journal of Medicinal Chemistry 43 (22): 4045–4050. doi:10.1021/jm000249r. PMID 11063601.
- ↑ Macor, J.; Mullen, G.; Verhoest, P.; Sampognaro, A.; Shepardson, B.; Mack, R. (2004). "A chiral synthesis of (−)-spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidin-2′-one]: A conformationally restricted analogue of acetylcholine that is a potent and selective α7 nicotinic receptor agonist". The Journal of Organic Chemistry 69 (19): 6493–6495. doi:10.1021/jo049404q. PMID 15357617.
- ↑ Levin, E. D.; Bettegowda, C.; Blosser, J.; Gordon, J. (1999). "AR-R 17779, an α7 nicotinic agonist, improves learning and memory in rats". Behavioural Pharmacology 10 (6–7): 675–680. doi:10.1097/00008877-199911000-00014. PMID 10780509.
- ↑ Van Kampen, M.; Selbach, K.; Schneider, R.; Schiegel, E.; Boess, F.; Schreiber, R. (2004). "AR-R 17779 improves social recognition in rats by activation of nicotinic α7 receptors". Psychopharmacology 172 (4): 375–383. doi:10.1007/s00213-003-1668-7. PMID 14727003.
- ↑ Grottick, A. J.; Trube, G.; Corrigall, W. A.; Huwyler, J.; Malherbe, P.; Wyler, R.; Higgins, G. A. (2000). "Evidence that nicotinic α7 receptors are not involved in the hyperlocomotor and rewarding effects of nicotine". The Journal of Pharmacology and Experimental Therapeutics 294 (3): 1112–1119. PMID 10945867.
- ↑ Van Maanen, M.; Lebre, M.; Van Der Poll, T.; Larosa, G.; Elbaum, D.; Vervoordeldonk, M.; Tak, P. (2009). "Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice". Arthritis and Rheumatism 60 (1): 114–122. doi:10.1002/art.24177. PMID 19116908.
 |
