Chemistry:Candocuronium iodide
This article may rely excessively on sources too closely associated with the subject, potentially preventing the article from being verifiable and neutral. (June 2014) (Learn how and when to remove this template message) |
This article may be too technical for most readers to understand. Please help improve it to make it understandable to non-experts, without removing the technical details. (September 2025) (Learn how and when to remove this template message) |
}}
 | |
| Clinical data | |
|---|---|
| Other names | Chandonium iodide; HS-310 |
| Pregnancy category |
|
| Routes of administration | IV |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
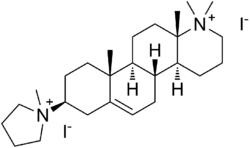
| Formula | C26H46I2N2 |
| Molar mass | 640.477 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Candocuronium iodide (INN; formerly chandonium iodide or HS-310)[1] is an aminosteroid neuromuscular-blocking drug that was investigated as a muscle relaxant for use in anesthesia. It acts by blocking the binding of the nicotinic acetylcholine receptor at the neuromuscular junction.[2] By blocking these receptors, it prevents acetylcholine from triggering muscle contraction, leading to muscle relaxation.
Medical use and discontinuation
Candocuronium was clinically evaluated in India for providing skeletal muscle relaxation during surgery, easing tracheal intubation, and assisting with mechanical ventilation.[3] Clinical studies reported a rapid onset of action with a short duration. Development was discontinued due to cardiovascular side effects, notably tachycardia.[3] Several studies suggested that the severity of these effects were similar to that of the clinically established neuromuscular blocker, pancuronium bromide.[4][5][6][7] Research indicated that candocuronium had minimal ganglion-blocking activity and higher potency than pancuronium.[1]
History and development
Rationale and design
The drug was developed in the laboratory of Harkishan Singh at Panjab University as part of a research program seeking a non-depolarizing neuromuscular blocker to replace the widely used depolarizing agent suxamethonium (succinylcholine).[8] The design of candocuronium places it in a series of mono- and bis-quaternary azasteroid. The approach adopted in its development used the rigid steroid skeleton as a spacer to hold two quaternary ammonium groups (inspired by the alkaloid malouetine), which incorporate fragments resembling choline or acetylcholine, at a specific distance.[8]
Synthesis and early analogs
The research program first produced HS-342, a bis-quaternary agent that was reportedly equipotent with tubocurarine and had one-third its duration of action. However, it was deemed unsuitable for further clinical evaluation.[9][10]
Subsequent chemical modifications of HS-342 led to the synthesis of two related derivatives: HS-310 (later named candocuronium) and HS-347.[1][8] HS-347, though equipotent with tubocurarine, was precluded from clinical trials because it exhibited considerable ganglion-blocking activity, which would potentially lead to undesirable autonomic side effects.[11][12]
Further modifications and legacy
H-310 did not achieve the desired clinical profile, which led to the continued modification of its structure, ultimately resulting in the creation of dihydrochandonium (HS-626). The new variant was an analog, and was reported to be a slightly better neuromuscular blocking profile and had no vagolytic effects.[13][14] However, this benefit was not considered significant enough to advance the compound to human trials.[15]
The discovery of candocuronium prompted further research into modifications of the androstane nucleus, particularly at the 3- and 16-positions, leading to the development of other agents considered for clinical testing.[16][17][18][19]
References
- ↑ 1.0 1.1 1.2 "Neuromuscular and other blocking actions of a new series of mono and bisquaternary aza steroids". The Journal of Pharmacy and Pharmacology 26 (11): 871–877. Nov 1974. doi:10.1111/j.2042-7158.1974.tb09195.x. PMID 4156557.
- ↑ "Actions of the muscle relaxant chandonium iodide on guinea-pig ileum and vas deferens preparations". The Journal of Pharmacy and Pharmacology 28 (8): 617–619. Aug 1976. doi:10.1111/j.2042-7158.1976.tb02812.x. PMID 11309.
- ↑ 3.0 3.1 "Catalyzing the Future of Medicinal Chemistry Research in India". Journal of Medicinal Chemistry 66 (16): 10868–10877. August 2023. doi:10.1021/acs.jmedchem.3c01304. PMID 37561395.
- ↑ "Comparative clinical evaluation of chandonium iodide and pancuronium bromide as muscle relaxant". Journal of Postgraduate Medicine 36 (2): 95–99. Apr 1990. PMID 2151453.
- ↑ "Clinical evaluation of chandonium iodide as muscle relaxant". The Indian Journal of Medical Research 87: 298–302. Mar 1988. PMID 3397166.
- ↑ "Clinical evaluation of chandonium iodide as a nondepolarising muscle relaxant". The Indian Journal of Medical Research 92: 367–370. Oct 1990. PMID 2148735.
- ↑ Suri YV (1984). Chandonium-iodide. New non-depolarising muscle relaxant. In: "Anaesthesiology. Clinical Pharmacology" Suri YV, Singh D (Eds.) New Delhi: Vani Educational Books; 28-35.
- ↑ 8.0 8.1 8.2 "Steroids and related studies. Part XXV. Chandonium iodide (17a-methyl-3β-pyrrolidino-17a-aza-D-homoandrost-5-ene dimethiodide) and other quaternary ammonium steroid analogues". Journal of the Chemical Society, Perkin Transactions 1 0 (12): 1475–1479. 1974. doi:10.1039/p19740001475. PMID 4472321.
- ↑ "Some actions of 4,17a-dimethyl-4,17a-diaza-D-homo-5alpha-androstane dimethiodide (HS-342), a new neuromuscular blocking drug". The Journal of Pharmacy and Pharmacology 25 (6): 441–446. Jun 1973. doi:10.1111/j.2042-7158.1973.tb09130.x. PMID 4146581.
- ↑ "The neuromuscular and other blocking actions of 4,17a-dimethyl-4,17a-diaza-d-homo-5 -androstane dimethiodide (HS-342) in the anaesthetized cat". European Journal of Pharmacology 22 (2): 129–134. May 1973. doi:10.1016/0014-2999(73)90002-2. PMID 4715215.
- ↑ "Some actions of chandonium iodide, a new short-acting muscle relaxant, in anaesthetized cats and on isolated muscle preparations". Clinical and Experimental Pharmacology & Physiology 2 (2): 159–170. Mar–Apr 1975. doi:10.1111/j.1440-1681.1975.tb01830.x. PMID 237641.
- ↑ "The effects of dihydrochandonium and other chandonium analogues on neuromuscular and autonomic transmission". The Journal of Pharmacy and Pharmacology 31 (8): 521–528. Aug 1979. doi:10.1111/j.2042-7158.1979.tb13576.x. PMID 39992.
- ↑ "Steroids and related studies. Part 44. 17a-Methyl-3β-(N-pyrrolidinyl)17a-aza-D-homo-5α-androstane bis(methiodide)(dihydrochandonium iodide) and certain other analogues of chandonium iodide". Journal of the Chemical Society, Perkin Transactions 1: 305–307. 1979. doi:10.1039/P19790000305.
- ↑ "Steroids and related studies. Part 48. A chandonium iodide analogue possessing an acetylcholine-like moiety". Journal of the Chemical Society, Perkin Transactions 1: 2451. 1979. doi:10.1039/p19790002451.
- ↑ "The neuromuscular and autonomic blocking effects of azasteroids containing choline or acetylcholine fragments". The Journal of Pharmacy and Pharmacology 33 (7): 451–457. Jul 1981. doi:10.1111/j.2042-7158.1981.tb13831.x. PMID 6115032.
- ↑ "Synthesis and neuromuscular blocking activity of 16β-piperidinosteroidal derivatives". European Journal of Medicinal Chemistry 36 (2): 195–202. Feb 2001. doi:10.1016/s0223-5234(00)01205-8. PMID 11311750.
- ↑ "Synthesis and neuromuscular blocking activity of 16β-N-methylpiperazino steroidal derivatives". European Journal of Medicinal Chemistry 37 (11): 901–908. Nov 2002. doi:10.1016/s0223-5234(02)01413-7. PMID 12446049.
- ↑ "Pharmacokinetics and disposition of chandonium iodide in rat". Indian Journal of Experimental Biology 23 (5): 253–257. May 1985. PMID 4077122.
- ↑ "Pharmacokinetics and disposition of chandonium iodide in monkey". Indian Journal of Experimental Biology 23 (5): 258–261. May 1985. PMID 4077123.
External links
- Neuromuscular+blocking+agents at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
