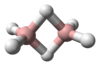Chemistry:Digallane
This article needs additional citations for verification. (October 2022) (Learn how and when to remove this template message) |
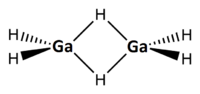
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
digallane(6)
| |||
| Other names
Di-μ-hydrido-tetrahydridodigallium
Gallane dimer | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| |||
| |||
| Properties | |||
| Ga 2H 6 | |||
| Molar mass | 145.494 g/mol | ||
| Appearance | White solid or colorless gas | ||
| Melting point | −50 °C (−58 °F; 223 K) (sublimes) | ||
| Boiling point | 0 °C (32 °F; 273 K) (decomposes) | ||
| Reacts to form gallium(III) hydroxide | |||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
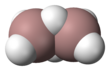
Digallane (systematically named digallane(6)) is an inorganic compound with the chemical formula GaH
2(H)
2GaH
2 (also written [{GaH
2(μ-H)}
2] or [Ga
2H
6]). It is the dimer of the monomeric compound gallane. The eventual preparation of the pure compound, reported in 1989,[1][2]
was hailed as a "tour de force."[3] Digallane had been reported as early as 1941 by Wiberg;[4] however, this claim could not be verified by later work by Greenwood and others.[5] This compound is a colorless gas that decomposes above 0 °C.
Preparation
A two-stage approach proved to be the key to successful synthesis of pure digallane. Firstly the dimeric monochlorogallane, (H
2GaCl)
2 (containing bridging chlorine atoms and thus formulated as (H
2Ga(μ-Cl))
2) was prepared via the hydrogenation of gallium trichloride, GaCl
3, with trimethylsilane, Me
3SiH. This step was followed by a further reduction with Li[GaH
4] (lithium tetrahydrogallate), solvent free, at −23 °C, to produce digallane, Ga
2H
6 in low yield.
- Ga
2Cl
6 + 4 Me
3SiH → (H
2GaCl)
2 + 4 Me
3SiCl - (H
2GaCl)
2 + 2 Li[GaH
4] → 2 Ga
2H
6 + 2 LiCl
Digallane is volatile and condenses at −50 °C into a white solid.
Structure and bonding
Electron diffraction measurements of the vapour at 255 K established that digallane is structurally similar to diborane with 2 bridging hydrogen atoms[2] (so-called three-center two-electron bonds). The terminal Ga-H bond length is 152 pm, the Ga-H bridging is 171 pm and the Ga-H-Ga angle is 98°. The Ga-Ga distance is 258 pm. The 1H NMR spectrum of a solution of digallane in toluene shows two peaks attributable to terminal and bridging hydrogen atoms.[2]
In the solid state, digallane appears to adopt a polymeric or oligomeric structure. The vibrational spectrum is consistent with tetramer (i.e. (GaH
3)
4).[2] The vibrational data indicate the presence of terminal hydride ligands. In contrast, the hydrogen atoms are all bridging in α-alane, a high-melting, relatively stable polymeric form of aluminium hydride wherein the aluminium centers are 6-coordinated. Digallane decomposes at ambient temperatures:
- Ga
2H
6 → 2 Ga + 3 H
2
References
- ↑ Anthony J. Downs; Michael J. Goode; Colin R. Pulham (1989). "Gallane at last!". Journal of the American Chemical Society 111 (5): 1936–1937. doi:10.1021/ja00187a090.
- ↑ 2.0 2.1 2.2 2.3 Pulham C.R.; Downs A.J.; Goode M.J; Rankin D.W.H. Roberson H.E. (1991). "Gallane: Synthesis, Physical and Chemical Properties, and Structure of the Gaseous Molecule Ga2H6 As Determined by Electron Diffraction". Journal of the American Chemical Society 113 (14): 5149–5162. doi:10.1021/ja00014a003.
- ↑ N.N. Greenwood (2001). "Main group element chemistry at the millennium". J. Chem. Soc., Dalton Trans. (14): 2055–2066. doi:10.1039/b103917m.
- ↑ Wiberg E.; Johannsen T. (1941). "Über einen flüchtigen Galliumwasserstoff der Formel Ga2H6 und sein Tetramethylderivat". Naturwissenschaften 29 (21): 320. doi:10.1007/BF01479551. Bibcode: 1941NW.....29..320W.
- ↑ Shriver, D. F.; Parry, R. W.; Greenwood, N. N.; Storr, A; Wallbridge, M. G. H. (1963). "Some Observations Relative to Digallane". Inorg. Chem. 2 (4): 867–868. doi:10.1021/ic50008a053.
 |