Chemistry:Diborane(4)
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Diborane(4)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| 24760 | |
PubChem CID
|
|
| |
| |
| Properties | |
| B2H4 | |
| Molar mass | 25.65 g·mol−1 |
| Related compounds | |
Related compounds
|
Bis(pinacolato)diboron Diboron tetrafluoride Tetrahydroxydiborane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
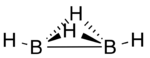
Diborane(4) is a transient inorganic compound with the chemical formula B2H4. Stable derivatives are known.
Diborane(4) has been produced by abstraction of two hydrogen atoms from diborane(6) using atomic fluorine and detected by photoionization mass spectrometry.[1] Computational studies predict a structure in which are two hydrogen atoms bridging the two boron atoms via three-centre two-electron bonds in addition to the 2-centre, 2-electron bond between the two boron atoms and one terminal hydrogen atom bonded to each boron atom.[2]
Several stable derivatives of diborane(4) have been reported.[3][4][5]
References
- ↑ Ruščic, B.; Schwarz, M.; Berkowitz, J. (1989). "Molecular structure and thermal stability of B2H4 and B2H+4 species". The Journal of Chemical Physics (AIP Publishing) 91 (8): 4576–4581. doi:10.1063/1.456745.
- ↑ Alkorta, Ibon; Soteras, Ignacio; Elguero, José; Del Beneb, Janet E. (23 June 2011). "The boron–boron single bond in diborane(4) as a non-classical electron donor for hydrogen bonding". Physical Chemistry Chemical Physics 13 (31): 14026–14032. doi:10.1039/C1CP20560A. PMID 21698334. Bibcode: 2011PCCP...1314026A. https://digital.csic.es/bitstream/10261/64351/1/accesoRestringido.pdf.
- ↑ Xie, Xiaochen; Haddow, Mairi F.; Mansell, Stephen M.; Norman, Nicholas C.; Russell, Christopher A. (2012). "Diborane(4) compounds with bidentate diamino groups". Dalton Transactions 41 (7): 2140–7. doi:10.1039/C2DT11936F. PMID 22187045.
- ↑ Wagner, Arne; Kaifer, Elisabeth; Himmel, Hans-Jörg (2012). "Diborane(4)–metal bonding: Between hydrogen bridges and frustrated oxidative addition". Chemical Communications 48 (43): 5277–9. doi:10.1039/C2CC31671D. PMID 22526934.
- ↑ Horn, Julian; Widera, Anna; Litters, Sebastian; Kaifer, Elisabeth; Himmel, Hans-Jörg (2018). "The proton affinity, HOMO energy and ionization energy of electron-rich sp3–sp3-hybridized diborane(4) compounds with bridging guanidinate substituents can be varied by substitution". Dalton Trans. 47 (6): 2009–2017. doi:10.1039/C7DT04433J. PMID 29345706.
 |








