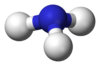
Chemistry:Indium trihydride
| |
| Names | |
|---|---|
| Systematic IUPAC name | |
| Other names
Indium(III) hydride
Indium trihydride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 163932 | |
PubChem CID
|
|
| |
| |
| Properties | |
| InH 3 | |
| Molar mass | 117.842 g/mol |
| Melting point | −90 °C (−130 °F; 183 K) (decomposes) |
| Structure | |
| Trigonal planar | |
| Dihedral | |
| Related compounds | |
Related metallanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Indium trihydride is an inorganic compound with the chemical formula (InH
3). It has been observed in matrix isolation and laser ablation experiments.[2][3] Gas phase stability has been predicted.[4] The infrared spectrum was obtained in the gas phase by laser ablation of indium in presence of hydrogen gas.[5] InH
3 is of no practical importance.
Chemical properties
Solid InH
3 is a three-dimensional network polymeric structure, where In atoms are connected by In-H-In bridging bonds, is suggested to account for the growth of broad infrared bands when samples of InH
3 and InD
3 produced on a solid hydrogen matrix are warmed.[5] Such a structure is known for solid AlH
3.[6] When heated above −90 °C, indium trihydride decomposes to produce indium–hydrogen alloy and elemental hydrogen. As of 2013, the only known method of synthesising indium trihydride is the autopolymerisation of indane below −90 °C.[clarification needed]
Other indium hydrides

3 and tricyclohexylphosphine.[7]
Several compounds with In-H bonds have been reported.[7] Examples of complexes with two hydride ligands replaced by other ligands are (K+
)
3[K((CH
3)
2SiO)
7]+
([InH(CH
2C(CH
3)
3)
3]−
)
4[8] and HIn(–C
6H
4–ortho-CH
2N(CH
3)
2)
2.
Although InH
3 is labile, adducts are known with the stoichiometry InH
3L
n (n = 1 or 2).[9]
1:1 amine adducts are made by the reaction of Li+
[InH
4]−
(lithium tetrahydridoindate(III)) with a trialkylammonium salt. The trimethylamine complex is only stable below −30 °C or in dilute solution. The 1:1 and 1:2 complexes with tricyclohexylphosphine (PCy
3) have been characterised crystallographically. The average In-H bond length is 168 pm.[7] Indium hydride is also known to form adducts with NHCs.[10]
References
- ↑ 1.0 1.1 "Indigane (CHEBI:30429)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:30429.
- ↑ Pullumbi, P.; Bouteiller, Y.; Manceron, L.; Mijoule, C. (July 1994). "Aluminium, gallium and indium trihydrides. An IR matrix isolation and ab initio study". Chemical Physics 185 (1): 25–37. doi:10.1016/0301-0104(94)00111-1. Bibcode: 1994CP....185...25P.
- ↑ Aldridge, S.; Downs, A. J. (2001). "Hydrides of the Main-Group Metals: New Variations on an Old Theme". Chemical Reviews 101 (11): 3305–65. doi:10.1021/cr960151d. PMID 11840988.
- ↑ Hunt, P.; Schwerdtfeger, P. (1996). "Are the Compounds InH3 and TlH3 Stable Gas Phase or Solid State Species?". Inorganic Chemistry 35 (7): 2085–2088. doi:10.1021/ic950411u.
- ↑ 5.0 5.1 Andrews, L.; Wang, X. (2004). "Infrared Spectra of Indium Hydrides in Solid Hydrogen and of Solid Indane". Angewandte Chemie International Edition 43 (13): 1706–1709. doi:10.1002/anie.200353216. PMID 15038043.
- ↑ Turley, J. W.; Rinn, H. W. (1969). "The Crystal Structure of Aluminum Hydride". Inorganic Chemistry 8 (1): 18–22. doi:10.1021/ic50071a005.
- ↑ 7.0 7.1 7.2 Jones, C. (2001). "The stabilisation and reactivity of indium trihydride complexes". Chemical Communications (22): 2293–2298. doi:10.1039/b107285b. ISSN 1359-7345. PMID 12240044.
- ↑ Rowen Churchill, M.; Lake, C. H.; Chao, S.-H. L.; Beachley, O. T. (1993). "Silicone grease as a precursor to a pseudo crown ether ligand: crystal structure of [K+]3[K(Me2SiO)7+][InH(CH2CMe3)3–]4". Journal of the Chemical Society, Chemical Communications 1993 (20): 1577–1578. doi:10.1039/C39930001577.
- ↑ Wang, X.; Andrews, L. (20 May 2004). "Infrared Spectra of Indium Hydrides in Solid Hydrogen and Neon". The Journal of Physical Chemistry A 108 (20): 4440–4448. doi:10.1021/jp037942l. Bibcode: 2004JPCA..108.4440W.
- ↑ Abernethy, C. D.; Cole, M. L.; Jones, C. (2000). "Preparation, Characterization, and Reactivity of the Stable Indium Trihydride Complex [InH3{IMes}]". Organometallics 19 (23): 4852–4857. doi:10.1021/om0004951.
 |