Chemistry:Plutonium(III) iodide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Appearance | green solid[1] |
| Density | 6.92 g·cm−3[2] |
| Melting point | 777 °C[1] |
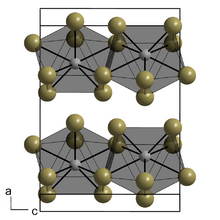
| Structure | |
| orthorhombic | |
| Ccmm (No. 63) | |
| Related compounds | |
Other anions
|
plutonium(III) hydride plutonium(III) fluoride plutonium(III) chloride plutonium(III) bromide |
Other cations
|
neptunium(III) iodide americium(III) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Plutonium(III) iodide is the iodide of plutonium with the chemical formula PuI3.
Preparation
Plutonium(III) iodide can be formed by the reaction of plutonium and mercury(II) iodide:[3]
- 2 Pu + 3 HgI
2 → 2 PuI
3 + 3 Hg
It can also be produced by reacting plutonium and hydrogen iodide at 450 °C. Even if there is only a small amount of oxygen and water in the reaction, the generated plutonium(III) iodide will immediately hydrolyze into plutonium iodide oxide.[3]
- 2 Pu + 6 HI → 2 PuI
3 + 3 H
2
Properties
Plutonium(III) iodide is a green solid with a melting point of 777 °C. It is orthorhombic (plutonium(III) bromide structure), space group Ccmm (No. 63), lattice parameters a = 433 pm, b = 1395 pm and c = 996 pm.[2]
References
- ↑ 1.0 1.1 Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils, ed., Inorganic Chemistry, San Diego/Berlin: Academic Press/De Gruyter, p. 1969, ISBN 0-12-352651-5
- ↑ 2.0 2.1 2.2 2.3 2.4 Gmelins Handbuch der anorganischen Chemie, System Nr. 71, Transurane, Teil C, S. 154–155.
- ↑ 3.0 3.1 Georg Brauer (Hrsg.), unter Mitarbeit von Marianne Baudler u. a.: Handbuch der Präparativen Anorganischen Chemie. 3., umgearbeitete Auflage. Band II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3, S. 1304.
External reading
- Clark, David L.; Hecker, Siegfried S.; Jarvinen, Gordon D.; Neu, Mary P. (2006), Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean, eds., "Plutonium" (in en), The Chemistry of the Actinide and Transactinide Elements (Dordrecht: Springer Netherlands): pp. 813–1264, doi:10.1007/1-4020-3598-5_7, ISBN 978-1-4020-3555-5, http://link.springer.com/10.1007/1-4020-3598-5_7, retrieved 2023-09-15
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

