Chemistry:Sodium aluminium hydride
| File:CaWO4.tif | |
| |
| Names | |
|---|---|
| IUPAC name
Sodium aluminium hydride
| |
| Systematic IUPAC name
Sodium alumanuide | |
| Other names
Sodium tetrahydroaluminate
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| AlH4Na | |
| Molar mass | 54.003 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 1.24 g/cm3 |
| Melting point | 183 °C (361 °F; 456 K) (decomposes) |
| Solubility | soluble in THF (16 g/100 mL at room temperature) |
| Hazards | |
| Safety data sheet | External MSDS |
| Flash point | −22 °C; −7 °F; 251 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
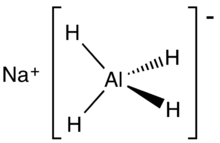
Sodium aluminium hydride or sodium alumanuide is an inorganic compound with the chemical formula NaAlH4. It is a white pyrophoric solid that dissolves in tetrahydrofuran (THF), but not in diethyl ether or hydrocarbons. It has been evaluated as an agent for the reversible storage of hydrogen and it is used as a reagent for the chemical synthesis of organic compounds. Similar to lithium aluminium hydride, it is a salt consisting of separated sodium cations and tetrahedral AlH−4 anions.[1]
Structure, preparation, and reactions
Sodium tetrahydroaluminate adopts the structure of (is isostructural with) calcium tungstate. As such, the tetrahedral AlH−4 centers are linked with eight-coordinate Na+ cations.[2] The compound is prepared from the elements under high pressures of H2 at 200 °C using triethylaluminium catalyst:[3]
- Na + Al + 2 H2 → NaAlH4
As a suspension in diethyl ether, it reacts with lithium chloride to give the popular reagent lithium aluminium hydride:
- LiCl + NaAlH4 → LiAlH4 + NaCl
The compound reacts rapidly, even violently, with protic reagents, such as water, as described in this idealized equation:
- 4 H2O + NaAlH4 → "NaAl(OH)4" + 4 H2
Applications
Hydrogen storage
Sodium alanate[4] has been explored for hydrogen storage in hydrogen tanks.[5] The relevant reactions are:
- 3 NaAlH4 → Na3AlH6+ 2 Al + 3 H2
- Na3AlH6 → 3 NaH + Al + 3/2 H2
Sodium tetrahydroaluminate can release up to 7.4 wt % of hydrogen when heated at 200 °C (392 °F). Absorption can be slow, with several minutes being required to fill a tank. Both release and uptake are catalysed by titanium.[6]
Reagent in organic chemistry
Sodium aluminium hydride is a strong reducing agent, very similar in reactivity to lithium aluminium hydride (LAH) and, to some extent, Diisobutylaluminium hydride (DIBAL) in organic reactions.[7] It is much more powerful reducing agent than sodium borohydride due to the weaker and more polar Al-H bond compared to the B-H bond. Like LAH, it reduces esters to alcohols.
Safety
Sodium aluminium hydride is highly flammable. It does not react in dry air at room temperature but is very sensitive to moisture. It ignites or explodes on contact with water.
See also
References
- ↑ J. W. Lauher, D. Dougherty P. J. Herley "Sodium tetrahydroaluminate" Acta Crystallogr. 1979, volume B35, pp.1454-1456. doi:10.1107/S0567740879006701
- ↑ Dougherty, D.; Herley, P. J.; Lauher, J. W. "Sodium tetrahydridoaluminate"Acta Crystallographica B (24,1968-38,1982) (1979) 35, p1454-p1456.
- ↑ Peter Rittmeyer, Ulrich Wietelmann "Hydrides" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_199
- ↑ Computational Study of Pristine and Titanium-doped Sodium Alanates for ...
- ↑ Zaluska, A.; Zaluski, L.; Ström-Olsen, J. O. (2000). "Sodium Alanates for Reversible Hydrogen Storage". Journal of Alloys and Compounds 298 (1–2): 125–134. doi:10.1016/S0925-8388(99)00666-0.
- ↑ "Researchers Solve Decade-Old Mystery of Hydrogen Storage Material". Phys.Org. 2008-02-27. http://phys.org/news123307288.html.
- ↑ Melinda Gugelchuk "Sodium Aluminum Hydride" Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley. doi:10.1002/047084289X.rs039
 |

