Chemistry:Titanium(III) iodide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Titanium(III) iodide
| |
| Other names
Titanium triiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| I3Ti | |
| Molar mass | 428.580 g·mol−1 |
| Appearance | black-violet solid |
| Density | 4.96 g·cm−3[1] |
| Related compounds | |
Other anions
|
Titanium(III) fluoride Titanium(III) bromide Titanium(III) chloride |
Other cations
|
Zirconium(III) iodide Hafnium(III) iodide |
Related compounds
|
Titanium(IV) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
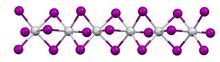
Titanium(III) iodide is an inorganic compound with the formula TiI3. It is a dark violet solid that is insoluble in solvents, except upon decomposition.
Preparation and structure
Titanium(III) iodide can be prepared by reaction of titanium with iodine:[2]
- [math]\displaystyle{ \mathrm{2 \ Ti + 3 \ I_2 \longrightarrow 2 \ TiI_3} }[/math]
It can also be obtained by reduction of TiI4, e.g., with aluminium.[3]
In terms of its structure, the compound exists as a polymer of face-sharing octahedra. Above 323 K, the Ti---Ti spacing are equal, but below that temperature, the material undergoes a phase transition. In the low temperature phase, the Ti---Ti contacts are alternating short and long. The low temperature structure is similar to that of molybdenum tribromide.[1]
References
- ↑ 1.0 1.1 Angelkort, Joachim; Schoenleber, Andreas; van Smaalen, Sander (2009). "Low- and High-Temperature Crystal Structures of TiI3". Journal of Solid State Chemistry 182: 525–53. doi:10.1016/j.jssc.2008.11.028..
- ↑ F. Hein, S. Herzog "Molybdenum(III) Bromide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1407.
- ↑ Catherine E. Housecroft, A. G. Sharpe (2005) (in German), Inorganic Chemistry, Pearson Education, pp. 601, ISBN 0-13039913-2, https://books.google.com/books?id=_1gFM51qpAMC
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

