Chemistry:Capsazepine
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
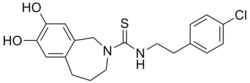
N-[2-(4-Chlorophenyl)ethyl]-7,8-dihydroxy-1,3,4,5-tetrahydro-2H-2-benzazepine-2-carbothioamide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H21ClN2O2S | |
| Molar mass | 376.9 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Capsazepine is a synthetic antagonist of capsaicin.[1] It is used as a biochemical tool in the study of TRPV ion channels.
Pharmacology
Capsazepine blocks the painful sensation of heat caused by capsaicin (the active ingredient of chilli pepper) which activates the TRPV1 ion channel. Capsazepine is therefore considered to be a TRPV1 antagonist. The TRPV1 channel functions as a pain and temperature sensor in mammalians. Capsazepine blocks the activation of TRPV1 channels by other chemicals, but not by other painful stimuli such as heat. Depending on the pharmacological assay, the IC50 is in the nanomolar to low micromolar range. In addition to its effects on TRPV1 channels, it was also shown to activate the noxious chemical sensor TRPA1 channel,[2] inhibit the cold activated TRPM8 channel,[3] voltage-activated calcium channels[4] and nicotinic acetylcholine receptors.[5] It mainly serves as a tool to study the TRPV1 ion channel.[6]
Development
Capsazepine was discovered by a research group working for Novartis.[1] Its synthesis and chemical properties were published in 1994. It was found by modification of the chemical backbone of capsaicin.[7]
Use in biotechnology
By incorporation of an azobenzene unit, a photoswitchable version of capsazepine (AC4) was developed in 2013 that allows for optical control of TRPV1 channels with light.[8][9]
See also
References
- ↑ 1.0 1.1 "Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin". British Journal of Pharmacology 107 (2): 544–52. October 1992. doi:10.1111/j.1476-5381.1992.tb12781.x. PMID 1422598.
- ↑ "Systemic desensitization through TRPA1 channels by capsazepine and mustard oil - a novel strategy against inflammation and pain". Scientific Reports 6: 28621. June 2016. doi:10.1038/srep28621. PMID 27356469.
- ↑ "Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay". British Journal of Pharmacology 141 (4): 737–45. February 2004. doi:10.1038/sj.bjp.0705652. PMID 14757700.
- ↑ "Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture". British Journal of Pharmacology 121 (7): 1461–7. August 1997. doi:10.1038/sj.bjp.0701272. PMID 9257928.
- ↑ "Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia". Neuroscience Letters 228 (1): 29–32. May 1997. doi:10.1016/S0304-3940(97)00358-3. PMID 9197280.
- ↑ "Current perspectives on the therapeutic utility of VR1 antagonists". Current Medicinal Chemistry 11 (24): 3185–202. December 2004. doi:10.2174/0929867043363686. PMID 15579007. http://www.bentham-direct.org/pages/content.php?CMC/2004/00000011/00000024/0003C.SGM.
- ↑ "The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin". Journal of Medicinal Chemistry 37 (13): 1942–54. June 1994. doi:10.1021/jm00039a006. PMID 8027976.
- ↑ "Optical control of TRPV1 channels". Angewandte Chemie 52 (37): 9845–8. September 2013. doi:10.1002/anie.201302530. PMID 23873837.
- ↑ Optical switches: Putting the fire out with light LMU Munich, 07/25/2013
 |

