Astronomy:Polyyne

A polyyne is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, (–C≡C–)
n with n greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature,[1] even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds (–CR=CR′–)
n with n greater than 1. They are also sometimes referred to as oligoynes,[2][needs IPA] or carbinoids after "carbyne" (–C≡C–)
∞, the hypothetical allotrope of carbon that would be the ultimate member of the series.[3][4] The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed.[5] Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts[6] would be called polyynes today.
The simplest polyyne is diacetylene or butadiyne, H–C≡C–C≡C–H. Along with cumulenes, polyynes are distinguished from other organic chains by their rigidity and high conductivity,[7] both of which make them promising as wires in molecular nanotechnology. Polyynes have been detected in interstellar molecular clouds where hydrogen is scarce.[citation needed]
Synthesis
The first reported synthesis of a polyyne was performed in 1869 by Carl Andreas Glaser (de), who observed that copper phenylacetylide (CuC≡C–C
6H
5) undergoes oxidative dimerization in the presence of air to produce diphenylbutadiyne (C
6H
5–C≡C–C≡C–C
6H
5).[4]
Interest in these compounds has stimulated research into their preparation by organic synthesis by several general routes. As a main synthetic tool usually acetylene homocoupling reactions like the Glaser coupling or its associated Elinton and Hay protocols are used.[8][4] Moreover, many of such procedures involve a Cadiot–Chodkiewicz coupling or similar reactions to unite two separate alkyne building-blocks or by alkylation of a pre-formed polyyne unit.[9] In addition to that, Fritsch–Buttenberg–Wiechell rearrangement was used as crucial step during the synthesis of the longest known polyyne (C
44).[10] An elimination of chlorovinylsilanes was used as a final step in the synthesis of the longest known phenyl end-capped polyynes.[11]
Organic and organosilicon polyynes
Using various techniques, polyynes H(–C≡C–)
nH with n up to 4 or 5 were synthesized during the 1950s.[12] Around 1971, T. R. Johnson and D. R. M. Walton developed the use of end-caps of the form –SiR
3, where R was usually an ethyl group, to protect the polyyne chain during the chain-doubling reaction using Hay's catalyst (a copper(I)–TMEDA complex).[12][13] With that technique they were able to obtain polyynes like (CH
3CH
2)
3Si(–C≡C–)
nSi(CH
2CH
3)
3 with n up to 8 in pure state, and with n up to 16 in solution.
Later Tykwinski and co-workers were able to obtain ((CH
3)
2CH)
3Si(–C≡C–)
nSi(CH(CH
3)
2)
3 polyynes with chain length up to C20.[14]
A polyyne compound with 10 acetylenic units (20 atoms), with the ends capped by Fréchet-type aromatic polyether dendrimers, was isolated and characterized in 2002.[2] Moreover, the synthesis of dicyanopolyynes with up to 8 acetylenic units was reported.[15] The longest phenyl end-capped polyynes were reported by Cox and co-workers in 2007.[11] As of 2010, the polyyne with the longest chain yet isolated had 22 acetylenic units (44 carbon atoms), end-capped with tris(3,5-di-t-butylphenyl)methyl groups.[10]
Alkynes with the formula H(–C≡C–)
nH and n from 2 to 6 can be detected in the decomposition products of partially oxidized copper(I) acetylide ((Cu+
)
2(−
C≡C−
) (an acetylene derivative known since 1856 or earlier) by hydrochloric acid. A "carbonaceous" residue left by the decomposition also has the spectral signature of (–C≡C–)
n chains.[16]

Organometallics
Organometallic polyynes capped with metal complexes are well characterized. As of the mid-2010s, the most intense research has concerned rhenium (Re(–C≡C–)
nRe, n = 3–10),[17] ruthenium (RuRu(–C≡C–)
nRuRu, n = 4–10),[18] iron (Fe(–C≡C–)
6Fe),[19] platinum (Pt(–C≡C–)
nPt, n = 8–14),[20] palladium (Ar(–C≡C–)
nPd, n = 3–5, Ar = aryl),[21] and cobalt (Co
3C(–C≡C–)
nCCo
3, n = 7–13)[22] complexes.
File:Organometallic polyynes.tif
Stability
Long polyyne chains are said to be inherently unstable in bulk because they can cross-link with each other exothermically.[5] Explosions are a real hazard in this area of research.[23] They can be fairly stable, even against moisture and oxygen, if the end hydrogen atoms are replaced with a suitably inert end-group, such as tert-butyl or trifluoromethyl.[24] Bulky end-groups, that can keep the chains apart, work especially well at stabilizing polyynes.[2] In 1995 the preparation of carbyne chains with over 300 carbon atoms was reported using this technique.[24] However the report has been contested by a claim that the detected molecules were fullerene-like structures rather than long polyynes.[5]
Polyyne chains have also been stabilised to heating by co-deposition with silver nanoparticles,[25] and by complexation with a mercury-containing tridentate Lewis acid to form layered adducts.[26] Long polyyne chains encapsulated in double-walled carbon nanotubes or in the form of rotaxanes[27] have also been shown to be stable.[28] Despite rather low stability of longer polyynes there are some examples of their use as synthetic precursors in organic and organometallic synthesis.[29]
Structure
Synthetic polyynes of the form R(–C≡C–)
nR, with n about 8 or more, often have a smoothly curved or helical backbone in the crystalline solid state, presumably due to crystal packing effects.[30] For example, when the cap R is triisopropylsilyl and n is 8, X-ray crystallography of the substance (a crystalline orange/yellow solid) shows the backbone bent by about 25–30 degrees in a broad arch, so that each C−C≡C angle deviates by 3.1 degrees from a straight line. This geometry affords a denser packing, with the bulky cap of an adjacent molecule nested into the concave side of the backbone. As a result, the distance between backbones of neighboring molecules is reduced to about 0.35 to 0.5 nm, near the range at which one expects spontaneous cross-linking. The compound is stable indefinitely at low temperature, but decomposes before melting. In contrast, the homologous molecules with n = 4 or n = 5 have nearly straight backbones that stay at least 0.5 to 0.7 nm apart, and melt without decomposing.[14]
Natural occurrence
Biological origins
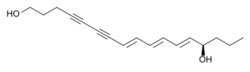
A wide range of organisms synthesize polyynes.[1][31] These chemicals have various biological activities, including as flavorings and pigments, chemical repellents and toxins, and potential application to biomedical research and pharmaceuticals. In plants, polyynes are found mainly in Asterids clade, especially in the sunflower, carrot, ginseng and bellflower families. However, they can also be found in some members of the tomato, olax, and sandalwood families.[32] The earliest polyyne to be isolated was dehydromatricaria ester (DME) in 1826; however, it was not fully characterized until later.[1][33]
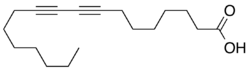
The simple fatty acid 8,10-octadecadiynoic acid is isolated from the root bark of the legume Paramacrolobium coeruleum of the family Caesalpiniaceae and has been investigated as a photopolymerizable unit in synthetic phospholipids.[9]
Thiarubrine B is the most prevalent among several related light-sensitive pigments that have been isolated from the Giant Ragweed (Ambrosia trifida), a plant used in herbal medicine. The thiarubrines have antibiotic, antiviral, and nematocidal activity, and activity against HIV-1 that is mediated by exposure to light.[34]
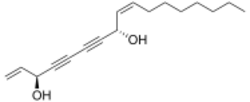
Polyynes such as falcarindiol can be found in Apiaceae vegetables like carrot, celery, fennel, parsley and parsnip where they show cytotoxic activities.[35] Aliphatic C
17-polyynes of the falcarinol type were described to act as metabolic modulators[36][37] and are studied as potential health-promoting nutraceuticals.[38] Falcarindiol is the main compound responsible for bitterness in carrots, and is the most active among several polyynes with potential anticancer activity found in Devil's club (Oplopanax horridus). Other polyynes from plants include oenanthotoxin and cicutoxin, which are poisons found in water dropwort (Oenanthe spp.) and water hemlock (Cicuta spp.).
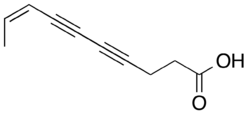
Ichthyothere is a genus of plants whose active constituent is a polyyne called ichthyothereol. This compound is highly toxic to fish and mammals.[39] Ichthyothere terminalis leaves have traditionally been used to make poisoned bait by indigenous peoples of the lower Amazon basin.[39]
Dihydromatricaria acid is a polyyne produced and secreted by soldier beetles as a chemical defense.[40]
In space
The octatetraynyl radicals and hexatriynyl radicals together with their ions are detected in space where hydrogen is rare.[41] Moreover, there have been claims[42] that polyynes have been found in astronomical impact sites on Earth as part of the mineral chaoite, but this interpretation has been contested.[43] See Astrochemistry.
See also
References
- ↑ 1.0 1.1 1.2 Minto RE; Blacklock BJ (July 2008). "Biosynthesis and function of polyacetylenes and allied natural products". Prog Lipid Res 47 (4): 233–306. doi:10.1016/j.plipres.2008.02.002. PMID 18387369.
- ↑ 2.0 2.1 2.2 Gibtner, Thomas; Hampel, Frank; Gisselbrecht, Jean-Paul; Hirsch, Andreas (2002). "End-cap stabilized oligoynes: Model compounds for the linear sp carbon allotrope carbyne". Chemistry: A European Journal 8 (2): 408–432. doi:10.1002/1521-3765(20020118)8:2<408::AID-CHEM408>3.0.CO;2-L. PMID 11843154.
- ↑ Heimann, R.B.; Evsyukov, S.E.; Kavan, L., eds (1999). Carbyne and carbynoid structures. Physics and Chemistry of Materials with Low-Dimensional Structures. 21. p. 452. ISBN 978-0-7923-5323-2.
- ↑ 4.0 4.1 4.2 Chalifoux, Wesley A.; Tykwinski, Rik R. (2009). "Synthesis of extended polyynes: Toward carbyne". Comptes Rendus Chimie 12 (3–4): 341–358. doi:10.1016/j.crci.2008.10.004. https://comptes-rendus.academie-sciences.fr/chimie/articles/10.1016/j.crci.2008.10.004/. In Avancés récentes en chimie des acétylènes – Recent advances in acetylene chemistry
- ↑ 5.0 5.1 5.2 Kroto, H. (November 2010). "Carbyne and other myths about carbon". RSC Chemistry World. http://www.rsc.org/chemistryworld/Issues/2010/November/CarbyneOtherMythsAboutCarbon.asp.
- ↑ Akagi, K.; Nishiguchi, M.; Shirakawa, H.; Furukawa, Y. et al. (1987). "One-dimensional conjugated carbyne — synthesis and properties". Synthetic Metals 17 (1–3): 557–562. doi:10.1016/0379-6779(87)90798-3.
- ↑ Bryce, Martin R. (2021). "A review of functional linear carbon chains (oligoynes, polyynes, cumulenes) and their applications as molecular wires in molecular electronics and optoelectronics". J. Mater. Chem. C 9 (33): 10524–10546. doi:10.1039/d1tc01406d. ISSN 2050-7526.
- ↑ Jevric, Martyn; Nielsen, Mogens Brøndsted (April 2015). "Synthetic Strategies for Oligoynes". Asian Journal of Organic Chemistry 4 (4): 286–295. doi:10.1002/ajoc.201402261.
- ↑ 9.0 9.1 Xu, Zhenchun; Byun, Hoe Sup; Bittman, Robert (1991). "Synthesis of photopolymerizable long-chain conjugated diacetylenic acids and alcohols from butadiyne synthons". J. Org. Chem. 56 (25): 7183–7186. doi:10.1021/jo00025a045.
- ↑ 10.0 10.1 Chalifoux, Wesley A.; Tykwinski, Rik R. (2010). "Synthesis of polyynes to model the sp-carbon allotrope carbyne". Nature Chemistry 2 (11): 967–971. doi:10.1038/nchem.828. PMID 20966954. Bibcode: 2010NatCh...2..967C.
- ↑ 11.0 11.1 Simpkins, Simon M. E.; Weller, Michael D.; Cox, Liam R. (2007). "β-Chlorovinylsilanes as masked alkynes in oligoyne assembly: synthesis of the first aryl-end-capped dodecayne". Chemical Communications (39): 4035–7. doi:10.1039/B707681A. PMID 17912407.
- ↑ 12.0 12.1 Eastmond, R.; Johnson, T.R.; Walton, D.R.M. (1972). "Silylation as a protective method for terminal alkynes in oxidative couplings: A general synthesis of the parent polyynes H(C≡C)nH (n = 4–10, 12)". Tetrahedron 28 (17): 4601–16. doi:10.1016/0040-4020(72)80041-3.
- ↑ Johnson, T.R.; Walton, D.R.M. (1972). "Silylation as a protective method in acetylene chemistry: Polyyne chain extensions using the reagents, Et3Si(C≡C)mH (m = 1, 2, 4) in mixed oxidative couplings". Tetrahedron 28 (20): 5221–36. doi:10.1016/S0040-4020(01)88941-9.
- ↑ 14.0 14.1 Eisler, Sara; Slepkov, Aaron D.; Elliott, Erin; Thanh Luu et al. (2005). "Polyynes as a model for carbyne: Synthesis, physical properties, and nonlinear optical response". Journal of the American Chemical Society 127 (8): 2666–76. doi:10.1021/ja044526l. PMID 15725024.
- ↑ Schermann, Günther; Grösser, Thomas; Hampel, Frank; Hirsch, Andreas (1997). "Dicyanopolyynes: A Homologous Series of End-Capped Linear sp Carbon" (in en). Chemistry – A European Journal 3 (7): 1105–1112. doi:10.1002/chem.19970030718. ISSN 1521-3765.
- ↑ Cataldo, Franco (1999). "From dicopper acetylide to carbyne". Polymer International 48 (1): 15–22. doi:10.1002/(SICI)1097-0126(199901)48:1<15::AID-PI85>3.0.CO;2-#.
- ↑ Dembinski, Roman; Bartik, Tamás; Bartik, Berit; Jaeger, Monika; Gladysz, J. A. (2000-02-01). "Toward Metal-Capped One-Dimensional Carbon Allotropes: Wirelike C6−C20 Polyynediyl Chains That Span Two Redox-Active (η5-C5Me5)Re(NO)(PPh3) Endgroups". Journal of the American Chemical Society 122 (5): 810–822. doi:10.1021/ja992747z. ISSN 0002-7863.
- ↑ Cao, Zhi; Xi, Bin; Jodoin, Diane S.; Zhang, Lei; Cummings, Steven P.; Gao, Yang; Tyler, Sarah F.; Fanwick, Phillip E. et al. (2014-08-27). "Diruthenium–Polyyn-diyl–Diruthenium Wires: Electronic Coupling in the Long Distance Regime". Journal of the American Chemical Society 136 (34): 12174–12183. doi:10.1021/ja507107t. ISSN 0002-7863. PMID 25116468.
- ↑ Sakurai, Aizoh; Akita, Munetaka; Moro-oka, Yoshihiko (1999-08-01). "Synthesis and Characterization of the Dodecahexaynediyldiiron Complex, Fp*−(C≡C)6−Fp* [Fp*= Fe(η5-C5Me5)(CO)2], the Longest Structurally Characterized Polyynediyl Complex". Organometallics 18 (16): 3241–3244. doi:10.1021/om990266i. ISSN 0276-7333.
- ↑ Zheng, Qinglin; Gladysz, J. A. (2005-08-01). "A Synthetic Breakthrough into an Unanticipated Stability Regime: Readily Isolable Complexes in which C16−C28 Polyynediyl Chains Span Two Platinum Atoms". Journal of the American Chemical Society 127 (30): 10508–10509. doi:10.1021/ja0534598. ISSN 0002-7863. PMID 16045336.
- ↑ Pigulski, Bartłomiej; Gulia, Nurbey; Szafert, Sławomir (2015-10-22). "Synthesis of Long, Palladium End-Capped Polyynes through the Use of Asymmetric 1-Iodopolyynes". Chemistry: A European Journal 21 (49): 17769–17778. doi:10.1002/chem.201502737. ISSN 1521-3765. PMID 26490174.
- ↑ Bruce, Michael I.; Zaitseva, Natasha N.; Nicholson, Brian K.; Skelton, Brian W.; White, Allan H. (2008-08-15). "Syntheses and molecular structures of some compounds containing many-atom chains end-capped by tricobalt carbonyl clusters". Journal of Organometallic Chemistry 693 (17): 2887–2897. doi:10.1016/j.jorganchem.2008.06.007.
- ↑ Baughman, R.H. (2006). "Dangerously Seeking Linear Carbon". Science 312 (5776): 1009–1110. doi:10.1126/science.1125999. PMID 16709775.
- ↑ 24.0 24.1 Lagow, R.J.; Kampa, J.J.; Han-Chao Wei; Battle, Scott L. et al. (1995). "Synthesis of linear acetylenic carbon: The "sp" carbon allotrope". Science 267 (5196): 362–7. doi:10.1126/science.267.5196.362. PMID 17837484. Bibcode: 1995Sci...267..362L.
- ↑ Casari, C. S. et al. (2007). "Stabilization of linear carbon structures in a solid Ag nanoparticle assembly". Applied Physics Letters 90 (1): 013111. doi:10.1063/1.2430676. Bibcode: 2007ApPhL..90a3111C.
- ↑ Gabbai, F. P.; Taylor, T. J. (March 24, 2006). "Supramolecular Stabilization of α,ω-Diphenylpolyynes by Complexation to the Tridentate Lewis Acid [o-C6F4Hg]3". Organometallics 25 (9): 2143–2147. doi:10.1021/om060186w.
- ↑ Movsisyan, Levon D.; Franz, Michael; Hampel, Frank; Thompson, Amber L.; Tykwinski, Rik R.; Anderson, Harry L. (2016). "Polyyne Rotaxanes: Stabilization by Encapsulation". Journal of the American Chemical Society 138 (4): 1366–1376. doi:10.1021/jacs.5b12049. PMID 26752712.
- ↑ Zhao, C.; Shinohara, H. (2011). "Growth of Linear Carbon Chains inside Thin Double-Wall Carbon Nanotubes". Journal of Physical Chemistry C 115 (27): 13166–13170. doi:10.1021/jp201647m.
- ↑ Pigulski, Bartłomiej; Gulia, Nurbey; Szafert, Sławomir (2019). "Reactivity of Polyynes: Complex Molecules from Simple Carbon Rods" (in en). European Journal of Organic Chemistry 2019 (7): 1420–1445. doi:10.1002/ejoc.201801350. ISSN 1099-0690.
- ↑ Szafert, Slawomir; Gladysz, J. A. (2006-11-01). "Update 1 of: Carbon in One Dimension: Structural Analysis of the Higher Conjugated Polyynes". Chemical Reviews 106 (11): PR1–PR33. doi:10.1021/cr068016g. ISSN 0009-2665. PMID 17100401.
- ↑ Annabelle, L.K.; Shi Shun; Tykwinski, Rik R. (2006). "Synthesis of Naturally Occurring Polyynes". Angewandte Chemie International Edition 45 (7): 1034–57. doi:10.1002/anie.200502071. PMID 16447152.
- ↑ Konovalov, D. A. (December 2014). "Polyacetylene Compounds of Plants of the Asteraceae Family (Review)". Pharmaceutical Chemistry Journal 48 (9): 613–631. doi:10.1007/s11094-014-1159-7. ISSN 0091-150X. http://link.springer.com/10.1007/s11094-014-1159-7. Retrieved 2020-01-07.
- ↑ Stavholt, K., and N. A. Sorensen. 1950. Studies relating to naturally-occurring Acetylene Compounds: V. Dehydro Matricaria Ester (Methyl n-decene-triynoate) from the Essential Oil of Artemisia vulgaris L. Acta Chemica Scandinavia 4.
- ↑ Block, Eric; Guo, Chuangxing; Thiruvazhi, Mohan; Toscano, Paul J. (1994). "Total Synthesis of Thiarubrine B [3-(3-Buten-1-ynyl)-6-(1,3-pentadiynyl)-1,2-dithiin], the Antibiotic Principle of Giant Ragweed (Ambrosia trifida)". J. Am. Chem. Soc. 116 (20): 9403–9404. doi:10.1021/ja00099a097.
- ↑ Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B. et al. (2005). "Polyacetylenes from the Apiaceae Vegetables Carrot, Celery, Fennel, Parsley, and Parsnip and Their Cytotoxic Activities". J. Agric. Food Chem. 53 (7): 2518–23. doi:10.1021/jf048041s. PMID 15796588.
- ↑ Atanasov, AG; Blunder, M; Fakhrudin, N; Liu, X; Noha, SM; Malainer, C; Kramer, MP; Cocic, A et al. (Apr 2013). "Polyacetylenes from Notopterygium incisum–new selective partial agonists of peroxisome proliferator-activated receptor-gamma". PLOS ONE 8 (4): e61755. doi:10.1371/journal.pone.0061755. PMID 23630612. Bibcode: 2013PLoSO...861755A.
- ↑ Ohnuma, T; Anan, E; Hoashi, R; Takeda, Y; Nishiyama, T; Ogura, K; Hiratsuka, A (2011). "Dietary diacetylene falcarindiol induces phase 2 drug-metabolizing enzymes andblocks carbon tetrachloride-induced hepatotoxicity in mice through suppression of lipid peroxidation". Biol Pharm Bull 34 (3): 371–8. doi:10.1248/bpb.34.371. PMID 21372387.
- ↑ Christensen, LP (Jan 2011). "Aliphatic C17-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family". Recent Pat Food Nutr Agric 3 (1): 64–77. doi:10.2174/2212798411103010064. PMID 21114468.
- ↑ 39.0 39.1 Cascon, Seiva C.; Mors, Walter B.; Tursch, Bernard M.; Aplin, Robin T.; Durham, Lois J. (1965). "Ichthyothereol and Its Acetate, the Active Polyacetylene Constituents of Ichthyothere terminalis (Spreng.) Malme, a Fish Poison from the Lower Amazon". Journal of the American Chemical Society 87 (22): 5237–5241. doi:10.1021/ja00950a044. ISSN 0002-7863. PMID 5844817.
- ↑ Eisner, Thomas; Eisner, Maria; Siegler, Melody (2005). "40. Chauliognathus lecontei (a soldier beetle)". Secret Weapons: Defenses of Insects, Spiders, Scorpions, and Other Many-legged Creatures. Harvard University Press. pp. 185–188. ISBN 9780674018822. https://archive.org/details/secretweaponsdef00eisn/page/185.
- ↑ Duley, W. W.; Hu, A. (2009). "Polyynes and interstellar carbon nanoparticles". Astrophys. J. 698 (1): 808–811. doi:10.1088/0004-637X/698/1/808. Bibcode: 2009ApJ...698..808D.
- ↑ El Goresy, A.; Donnay, G. (1968). "A New Allotropic Form of Carbon from the Ries Crater". Science 151 (3839): 363–364. doi:10.1126/science.161.3839.363. PMID 17776738. Bibcode: 1968Sci...161..363E.
- ↑ Smith, P. P. K.; Busek, P. R. (1982). "Carbyne Forms of Carbon: Do They Exist?". Science 216 (4549): 984–986. doi:10.1126/science.216.4549.984. PMID 17809068. Bibcode: 1982Sci...216..984S.
 |