Biology:Gastrin
 Generic protein structure example |
| Gastrin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Gastrin | ||||||||
| Pfam | PF00918 | ||||||||
| InterPro | IPR001651 | ||||||||
| PROSITE | PDOC00232 | ||||||||
| |||||||||
Gastrin is a peptide hormone that stimulates secretion of gastric acid (HCl) by the parietal cells of the stomach and aids in gastric motility. It is released by G cells in the pyloric antrum of the stomach, duodenum, and the pancreas.
Gastrin binds to cholecystokinin B receptors to stimulate the release of histamines in enterochromaffin-like cells, and it induces the insertion of K+/H+ ATPase pumps into the apical membrane of parietal cells (which in turn increases H+ release into the stomach cavity). Its release is stimulated by peptides in the lumen of the stomach.
Physiology
Genetics
In humans, the GAS gene is located on the long arm of the seventeenth chromosome (17q21).[1]
Synthesis
Gastrin is a linear peptide hormone produced by G cells of the duodenum and in the pyloric antrum of the stomach. It is secreted into the bloodstream. The encoded polypeptide is preprogastrin, which is cleaved by enzymes in posttranslational modification to produce progastrin (an intermediate, inactive precursor) and then gastrin in various forms, primarily the following three:
- gastrin-34 ("big gastrin")
- gastrin-17 ("little gastrin")
- gastrin-14 ("minigastrin")
Also, pentagastrin is an artificially synthesized, five amino acid sequence identical to the last five amino acid sequence at the C-terminus end of gastrin. The numbers refer to the amino acid count.
Release
Gastrin is released in response to certain stimuli. These include:
- stomach antrum distension
- vagal stimulation (mediated by the neurocrine bombesin, or GRP in humans)
- the presence of partially digested proteins, especially amino acids, in the stomach. Aromatic amino acids are particularly powerful stimuli for gastrin release.[2]
- hypercalcemia (via calcium-sensing receptors[3])
Gastrin release is inhibited by:[4][5]
- the presence of acid (primarily the secreted HCl) in the stomach (a case of negative feedback)
- somatostatin also inhibits the release of gastrin, along with secretin, GIP (gastroinhibitory peptide), VIP (vasoactive intestinal peptide), glucagon and calcitonin.
Function
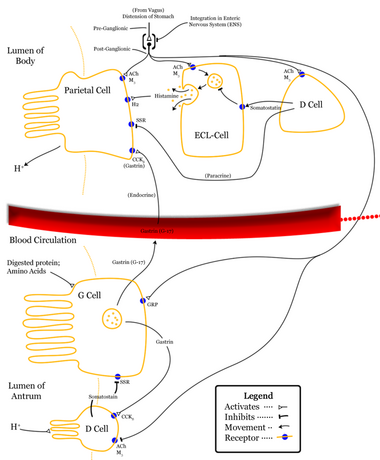
The presence of gastrin stimulates parietal cells of the stomach to secrete hydrochloric acid (HCl)/gastric acid. This is done both directly on the parietal cell [failed verification] and indirectly via binding onto CCK2/gastrin receptors on ECL cells in the stomach, which then responds by releasing histamine, which in turn acts in a paracrine manner on parietal cells stimulating them to secrete H+ ions. This is the major stimulus for acid secretion by parietal cells.[6]
Along with the above-mentioned function, gastrin has been shown to have additional functions as well:
- Stimulates parietal cell maturation and fundal growth.
- Causes chief cells to secrete pepsinogen, the zymogen (inactive) form of the digestive enzyme pepsin.
- Increases antral muscle mobility and promotes stomach contractions.
- Strengthens antral contractions against the pylorus, and relaxes the pyloric sphincter, which increases the rate of gastric emptying.[7]
- Plays a role in the relaxation of the ileocecal valve.[8]
- Induces pancreatic secretions and gallbladder emptying.[9]
- May impact lower esophageal sphincter (LES) tone, causing it to contract,[10] - although pentagastrin, rather than endogenous gastrin, may be the cause.[11]
- Gastrin contributes to the gastrocolic reflex.
Factors influencing secretion
Factors influencing secretion of gastrin can be divided into 2 categories:[12]
Physiologic
Gastric lumen
- Stimulatory factors: dietary protein and amino acids (meat), hypercalcemia. (i.e. during the gastric phase)
- Inhibitory factor: acidity (pH below 3) - a negative feedback mechanism, exerted via the release of somatostatin from δ cells in the stomach, which inhibits gastrin and histamine release.
Paracrine
- Stimulatory factor: bombesin or gastrin-releasing peptide (GRP)
- Inhibitory factor: somatostatin - acts on somatostatin-2 receptors on G cells. in a paracrine manner via local diffusion in the intercellular spaces, but also systemically through its release into the local mucosal blood circulation; it inhibits acid secretion by acting on parietal cells.
Nervous
- Stimulatory factors: Beta-adrenergic agents, cholinergic agents, gastrin-releasing peptide (GRP)
- Inhibitory factor: Enterogastric reflex
Circulation
- Stimulatory factor: gastrin
- Inhibitory factors:gastric inhibitory peptide (GIP), secretin, somatostatin, glucagon, calcitonin
Pathophysiologic
Paraneoplastic
- Gastrinoma paraneoplastic oversecretion (see Role in disease)
Role in disease
In the Zollinger–Ellison syndrome, gastrin is produced at excessive levels, often by a gastrinoma gastrin-producing tumor, mostly benign of the duodenum or the pancreas. To investigate for hypergastrinemia high blood levels of gastrin, a "pentagastrin test" can be performed.[13]
In autoimmune gastritis, the immune system attacks the parietal cells leading to hypochlorhydria low stomach acid secretion. This results in an elevated gastrin level in an attempt to compensate for increased pH in the stomach. Eventually, all the parietal cells are lost and achlorhydria results leading to a loss of negative feedback on gastrin secretion. Plasma gastrin concentration is elevated in virtually all individuals with mucolipidosis type IV (mean 1507 pg/mL; range 400-4100 pg/mL) (normal 0-200 pg/mL) secondary to a constitutive achlorhydria. This finding facilitates the diagnosis of patients with this neurogenetic disorder.[14] Additionally, elevated gastrin levels may be present in chronic gastritis resulting from H pylori infection.[15]
History
Its existence was first suggested in 1905 by the British physiologist John Sydney Edkins,[16][17] and gastrins were isolated in 1964 by Hilda Tracy and Roderic Alfred Gregory at the University of Liverpool.[18] In 1964 the structure of gastrin was determined.[19]
References
- ↑ "The genes for human gastrin and cholecystokinin are located on different chromosomes". Human Genetics 73 (1): 77–80. May 1986. doi:10.1007/BF00292669. PMID 3011648.
- ↑ Blanco, Antonio; Blanco, Gustavo (2017), "Biochemical Bases of Endocrinology (II) Hormones and Other Chemical Intermediates", Medical Biochemistry (Elsevier): pp. 573–644, doi:10.1016/b978-0-12-803550-4.00026-4, ISBN 9780128035504
- ↑ "Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion". Proceedings of the National Academy of Sciences of the United States of America 107 (41): 17791–6. October 2010. doi:10.1073/pnas.1009078107. PMID 20876097. Bibcode: 2010PNAS..10717791F.
- ↑ "Somatostatin is an essential paracrine link in acid inhibition of gastrin secretion". Digestion 51 (2): 95–102. 1992. doi:10.1159/000200882. PMID 1354190.
- ↑ "Effects of somatostatin and acid on inhibition of gastrin release in newborn rats". Endocrinology 114 (3): 743–6. March 1984. doi:10.1210/endo-114-3-743. PMID 6141932. http://endo.endojournals.org/cgi/content/abstract/114/3/743. Retrieved 2011-05-17.
- ↑ Lindström, E.; Chen, D.; Norlén, P.; Andersson, K.; Håkanson, R. (2001). "Control of gastric acid secretion:the gastrin-ECL cell-parietal cell axis". Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology 128 (3): 505–514. doi:10.1016/s1095-6433(00)00331-7. ISSN 1095-6433. PMID 11246041.
- ↑ Tortora, G. J., & Grabowski, S. R. (1996). Principles of anatomy and physiology. New York, NY: HarperCollins College. 14th Ed. Pg 906
- ↑ "Effects of gastrin-releasing peptide (GRP) on the mechanical activity of the human ileocaecal region in vitro". Neurogastroenterology and Motility 9 (4): 265–70. December 1997. doi:10.1046/j.1365-2982.1997.d01-59.x. PMID 9430795.
- ↑ "Effect of gastrin on pancreatic enzyme secretion and gallbladder emptying in man". Gastroenterology 71 (3): 409–11. September 1976. doi:10.1016/S0016-5085(76)80445-3. PMID 950091.
- ↑ "Gastrin and lower esophageal sphincter tone". Archives of Internal Medicine 138 (2): 196. February 1978. doi:10.1001/archinte.138.2.196. PMID 626547.
- ↑ "Lower oesophageal sphincter response to gastrin--pharmacological or physiological?". Gut 19 (2): 99–102. February 1978. doi:10.1136/gut.19.2.99. PMID 631634.
- ↑ Indu Khurana (2006). Textbook medical physiology. New Delhi: Reed Elsevier India. p. 605. ISBN 978-8181478504. OCLC 968478170.
- ↑ Baron, J. H. (1978) (in en-gb). Clinical Tests of Gastric Secretion. doi:10.1007/978-1-349-03188-7. ISBN 978-1-349-03190-0.
- ↑ "Constitutive achlorhydria in mucolipidosis type IV". Proceedings of the National Academy of Sciences of the United States of America 95 (3): 1207–12. February 1998. doi:10.1073/pnas.95.3.1207. PMID 9448310. Bibcode: 1998PNAS...95.1207S.
- ↑ "Review Article: Strategies to Determine Whether Hypergastrinaemia Is Due to Zollinger-Ellison Syndrome Rather Than a More Common Benign Cause". http://www.medscape.com/viewarticle/703619_5.
- ↑ "The chemical mechanism of gastric secretion". The Journal of Physiology 34 (1–2): 133–44. March 1906. doi:10.1113/jphysiol.1906.sp001146. PMID 16992839.
- ↑ "The pivotal role of John S. Edkins in the discovery of gastrin". World Journal of Surgery 21 (2): 226–34. February 1997. doi:10.1007/s002689900221. PMID 8995084.
- ↑ "The constitution and properties of two gastrins extracted from hog antral mucosa: Part I the isolation of two gastrins from hog antral mucosa". Gut 5 (2): 103–107. 1964. doi:10.1136/gut.5.2.103. PMID 14159395.
- ↑ "The antral hormone gastrin. Structure of gastrin.". Nature 204 (4962): 931–3. December 1964. doi:10.1038/204931a0. PMID 14248711. Bibcode: 1964Natur.204..931G.
Further reading
- "Gastrin, CCK, signaling, and cancer". Annual Review of Physiology 63: 49–76. 2001. doi:10.1146/annurev.physiol.63.1.49. PMID 11181948.
- "Clinical endocrinology and metabolism. Gastrin". Best Practice & Research. Clinical Endocrinology & Metabolism 18 (4): 555–68. December 2004. doi:10.1016/j.beem.2004.07.003. PMID 15533775.
- "Sporadic versus hereditary gastrinomas of the duodenum and pancreas: distinct clinico-pathological and epidemiological features". World Journal of Gastroenterology 12 (34): 5440–6. September 2006. doi:10.3748/wjg.v12.i34.5440. PMID 17006979.
- "Interaction of synthetic human big gastrin with blood proteins of man and animals". Acta Hepato-Gastroenterologica 26 (2): 154–9. April 1979. PMID 463490.
- "[Gastrointestinal hormones. I. Hormones of the gastrin group]". Zeitschrift für Gastroenterologie 15 (4): 264–76. April 1977. PMID 871064.
- "Purification and structural determination of urinary NH2-terminal big gastrin fragments". Biochemical and Biophysical Research Communications 160 (3): 1364–70. May 1989. doi:10.1016/S0006-291X(89)80154-8. PMID 2730647.
- "Degradation of human gastrin and CCK by endopeptidase 24.11: differential behaviour of the sulphated and unsulphated peptides". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 996 (1–2): 82–8. June 1989. doi:10.1016/0167-4838(89)90098-8. PMID 2736261.
- "The genes for human gastrin and cholecystokinin are located on different chromosomes". Human Genetics 73 (1): 77–80. May 1986. doi:10.1007/BF00292669. PMID 3011648.
- "Expression of human gastrin gene in normal and gastrinoma tissues". Gene 50 (1–3): 345–52. 1986. doi:10.1016/0378-1119(86)90338-0. PMID 3034736.
- "Aminoacid constitution of two gastrins isolated from Zollinger-Ellison tumour tissue". Gut 10 (8): 603–8. August 1969. doi:10.1136/gut.10.8.603. PMID 5822140.
- "Structures of human gastrins I and II". Nature 209 (5023): 583–5. February 1966. doi:10.1038/209583b0. PMID 5921183.
- "Structural analysis of the gene encoding human gastrin: the large intron contains an Alu sequence". Proceedings of the National Academy of Sciences of the United States of America 81 (15): 4662–6. August 1984. doi:10.1073/pnas.81.15.4662. PMID 6087340. Bibcode: 1984PNAS...81.4662I.
- "Structure of a human gastrin gene". Proceedings of the National Academy of Sciences of the United States of America 81 (4): 1067–9. February 1984. doi:10.1073/pnas.81.4.1067. PMID 6322186. Bibcode: 1984PNAS...81.1067W.
- "Molecular cloning of the human gastrin gene". Nucleic Acids Research 11 (23): 8197–203. December 1983. doi:10.1093/nar/11.23.8197. PMID 6324077.
- "Molecular cloning of human gastrin cDNA: evidence for evolution of gastrin by gene duplication". Proceedings of the National Academy of Sciences of the United States of America 80 (10): 2866–9. May 1983. doi:10.1073/pnas.80.10.2866. PMID 6574456. Bibcode: 1983PNAS...80.2866B.
- "Molecular cloning of human gastrin precursor cDNA". Gene 26 (1): 53–7. December 1983. doi:10.1016/0378-1119(83)90035-5. PMID 6689486.
- "Molecular cloning and sequencing of the murine gastrin gene". Biochemical and Biophysical Research Communications 216 (1): 34–41. November 1995. doi:10.1006/bbrc.1995.2588. PMID 7488110.
- "Post-poly(Glu) cleavage and degradation modified by O-sulfated tyrosine: a novel post-translational processing mechanism". The EMBO Journal 14 (2): 389–96. January 1995. doi:10.1002/j.1460-2075.1995.tb07013.x. PMID 7530658.
- "Identification of gastrin component I as gastrin-71. The largest possible bioactive progastrin product". European Journal of Biochemistry 223 (3): 765–73. August 1994. doi:10.1111/j.1432-1033.1994.tb19051.x. PMID 8055952.
- "Post-translational processing of progastrin: inhibition of cleavage, phosphorylation and sulphation by brefeldin A". The Biochemical Journal 295 (Pt 3): 813–9. November 1993. doi:10.1042/bj2950813. PMID 8240296.
External links
- Overview at colostate.edu
- Nosek, Thomas M.. "Section 6/6ch4/s6ch4_14". Essentials of Human Physiology. http://humanphysiology.tuars.com/program/section6/6ch4/s6ch4_14.htm.
 |
