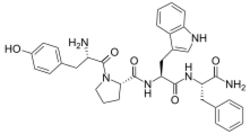
Chemistry:Endomorphin-1
| |

| |
| Names | |
|---|---|
| IUPAC name
L-Tyrosyl-L-prolyl-L-tryptophyl-L-phenylalaninamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C34H38N6O5 | |
| Molar mass | 610.703 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Endomorphin-1 (EM-1) (amino acid sequence Tyr-Pro-Trp-Phe-NH2) is an endogenous opioid peptide and one of the two endomorphins.[1] It is a high affinity, highly selective agonist of the μ-opioid receptor, and along with endomorphin-2 (EM-2), has been proposed to be the actual endogenous ligand of the μ-receptor.[1][2][3][4] EM-1 produces analgesia in animals and is equipotent with morphine in this regard.[3] The gene encoding for EM-1 has not yet been identified,[4] and it has been suggested that endomorphins could be synthesized by an enzymatic, non-ribosomal mechanism.[5]
By combining N-terminal guadino modifications, a new class of endonmorphin-1 was synthesized, the range of their bioactivities were measured by radioligand binding assay in order to conclude its potency as an opioid.[6] Endomorphin-1 has high affinity and specificity for opioid receptors for behavioral, physiological and pharmacological assays, it is also a potent analgesic agent which brings effects on cardiovascular, respiratory and gastrointestinal functions as well as in immune system responses.[citation needed] This endogenous opioid peptide can help with neuropathic pain without having the common side effects many neuropathic drugs caused which produces constipation. To make this drug side effect-free, a modification at the N-terminus by 2-aminodecainoic acid is made which in term showed an improve in the drug's metabolic stability along with improving its membrane permeability, while holding its high receptor binding affinity, helping the drug act as a potent agonist[7]
See also
References
- ↑ 1.0 1.1 Central Neural States Relating Sex and Pain. JHU Press. 3 April 2002. pp. 67–. ISBN 978-0-8018-6827-6. https://books.google.com/books?id=4KeQEC4nHSkC&pg=PA67.
- ↑ Neurogastroenterology - From the Basics to the Clinics. Springer Science & Business Media. 31 May 2000. pp. 76–. ISBN 978-0-7923-8757-2. https://books.google.com/books?id=ghjN0L47QbwC&pg=PA76.
- ↑ 3.0 3.1 Pain and Neurogenic Inflammation. Springer Science & Business Media. 1999. pp. 28–. ISBN 978-3-7643-5875-4. https://books.google.com/books?id=6TNSAeTpkUMC&pg=PA28.
- ↑ 4.0 4.1 Encyclopedia of Molecular Pharmacology. Springer Science & Business Media. 14 August 2008. pp. 904–. ISBN 978-3-540-38916-3. https://books.google.com/books?id=fGe6NDIGQpsC&pg=PA904.
- ↑ "Search of the human proteome for endomorphin-1 and endomorphin-2 precursor proteins". Life Sciences 81 (23–24): 1593–1601. November 2007. doi:10.1016/j.lfs.2007.09.025. PMID 17964607.
- ↑ "BrowZine". https://browzine.com/libraries/1453/journals/5448/issues/322551099.
- ↑ "Lipo-endomorphin-1 derivatives with systemic activity against neuropathic pain without producing constipation". PLOS ONE 7 (8): e41909. 2012-08-17. doi:10.1371/journal.pone.0041909. PMID 22912681. Bibcode: 2012PLoSO...741909V.
 |
