Chemistry:Beta-Endorphin
| |

| |
| Names | |
|---|---|
| IUPAC name
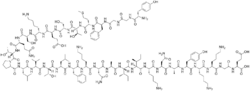
L-Tyrosylglycylglycyl-L-phenylalanyl-L-methionyl-L-threonyl-L-seryl-L-glutaminyl-L-lysyl-L-seryl-L-glutaminyl-L-threonyl-L-prolyl-L-leucyl-L-valyl-L-threonyl-L-leucyl-L-phenylalanyl-L-lysyl-L-asparaginyl-L-alanyl-L-isoleucyl-L-isoleucyl-L-lysyl-L-asparaginyl-L-alanyl-L-tyrosyl-L-lysyl-L-lysylglycyl-L-glutamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C158H251N39O46S | |
| Molar mass | 3465.03 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
beta-Endorphin (β-endorphin) is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system.[1] It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.[2]
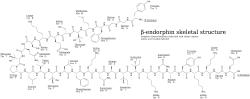
The amino acid sequence is: Tyr-Gly-Gly-Phe-Met-Thr-Ser-Glu-Lys-Ser-Gln-Thr-Pro-Leu-Val-Thr-Leu-Phe-Lys-Asn-Ala-Ile-Ile-Lys-Asn-Ala-Tyr-Lys-Lys-Gly-Glu (31 amino acids).[1][3] The first 16 amino acids are identical to α-endorphin. β-Endorphin is considered to be a part of the endogenous opioid and endorphin classes of neuropeptides;[1] all of the established endogenous opioid peptides contain the same N-terminal amino acid sequence, Tyr-Gly-Gly-Phe, followed by either -Met or -Leu.[1]
Function of β-endorphin has been known to be associated with hunger, thrill, pain, maternal care, sexual behavior, and reward cognition. In the broadest sense, β-endorphin is primarily utilized in the body to reduce stress and maintain homeostasis. In behavioral research, studies have shown that β-endorphin is released via volume transmission into the ventricular system in response to a variety of stimuli, and novel stimuli in particular.[4]
Formation and structure
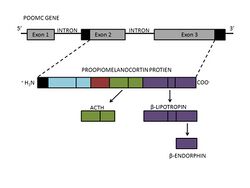
β-Endorphin is found in neurons of the hypothalamus, as well as the pituitary gland. It is derived from β-lipotropin, which is produced in the pituitary gland from a larger peptide precursor, proopiomelanocortin (POMC).[5] POMC is cleaved into two neuropeptides, adrenocorticotropic hormone (ACTH) and β-lipotropin.[6] The formation of β-endorphin is then the result of cleavage of the C-terminal region of β-lipotropin, producing a 31 amino acid-long neuropeptide with an alpha-helical secondary structure. However, POMC also gives rise to other peptide hormones, including α- and γ-melanocyte-stimulating hormone (MSH), resulting from intracellular processing by internal enzymes known as prohormone convertases.
A significant factor that differentiates β-endorphin from other endogenous opioids is its high affinity for and lasting effect on μ-opioid receptors.[5] The structure of β-endorphin in part accounts for this through its resistance to proteolytic enzymes, as its secondary structure makes it less vulnerable to degradation.[5]
Function and effects
β-Endorphin function is said to be divided into two main categories: local function and global function. Global function of β-endorphin is related to decreasing bodily stress and maintaining homeostasis resulting in pain management, reward effects, and behavioral stability. β-Endorphin in global pathways diffuse to different parts of the body through cerebral spinal fluid in the spinal cord, allowing for β-endorphin release to affect the peripheral nervous system. Localized function of β-endorphin results in release of β-endorphin in different brain regions such as the amygdala or the hypothalamus.[4] The two main methods by which β-endorphin is utilized in the body are peripheral hormonal action[7] and neuroregulation. β-endorphin and other enkephalins are often released with ACTH to modulate hormone system functioning. Neuroregulation by β-endorphin occurs through interference with the function of another neuropeptide, either by direct inhibition of neuropeptide release or induction of a signaling cascade that reduces a neuropeptide's effects.[6]
Opioid agonist
β-Endorphin is an agonist of the opioid receptors; it preferentially binds to the μ-opioid receptor.[1] Evidence suggests that it serves as a primary endogenous ligand for the μ-opioid receptor,[1][8] the same receptor to which the chemicals extracted from opium, such as morphine, derive their analgesic properties. β-Endorphin has the highest binding affinity of any endogenous opioid for the μ-opioid receptor.[1][5][8] Opioid receptors are a class of G-protein coupled receptors, such that when β-endorphin or another opioid binds, a signaling cascade is induced in the cell.[9] Acytelation of the N-terminus of β-endorphin, however, inactivates the neuropeptide, preventing it from binding to its receptor.[5] The opioid receptors are distributed throughout the central nervous system and within the peripheral tissue of neural and non-neural origin. They are also located in high concentrations in the Periaqueductal gray, Locus coeruleus, and the Rostral ventromedial medulla.[10]
Voltage-dependent calcium channels (VDCCs) are important membrane proteins that mediate the depolarization of neurons, and play a major role in promoting the release of neurotransmitters. When endorphin molecules bind to opioid receptors, G proteins activate and dissociate into their constituent Gα and Gβγ sub-units. The Gβγ sub-unit binds to the intracellular loop between the two trans-membrane helices of the VDCC. When the sub-unit binds to the voltage-dependent calcium channel, it produces a voltage-dependent block, which inhibits the channel, preventing the flow of calcium ions into the neuron. Embedded in the cell membrane is also the G protein-coupled inwardly-rectifying potassium channel. When a Gβγ or Gα(GTP) molecule binds to the C-terminus of the potassium channel, it becomes active, and potassium ions are pumped out of the neuron.[11][12] The activation of the potassium channel and subsequent deactivation of the calcium channel causes membrane hyperpolarization. This is when there is a change in the membrane's potential, so that it becomes more negative. The reduction in calcium ions causes a reduction neurotransmitter release because calcium is essential for this event to occur.[13] This means that neurotransmitters such as glutamate and substance P cannot be released from the presynaptic terminal of the neurons. These neurotransmitters are vital in the transmission of pain, and as β-Endorphin reduces the release of these substances, there is a strong analgesic effect.
Pain management
β-Endorphin has been primarily studied for its influence on nociception (i.e., pain perception). β-endorphin modulates pain perception both in the central nervous system and the peripheral nervous system. When pain is perceived, pain receptors (nociceptors) send signals to the dorsal horn of the spinal cord and then up to the hypothalamus through the release of a neuropeptide called substance P.[6][4][14][15] In the peripheral nervous system, this signal causes the recruitment of T-lymphocytes, white blood cells of the immune system, to the area where pain was perceived.[15] T-lymphocytes release β-endorphin in this localized region, allowing it to bind to opioid receptors, causing direct inhibition of substance P.[15][16] In the central nervous system, β-endorphin binds to opioid receptors in the dorsal root and inhibits the release of substance P in the spinal cord, reducing the number of excitatory pain signals sent to the brain.[15][14] The hypothalamus responds to the pain signal by releasing β-endorphin through the periaqueductal grey network, which mainly acts to inhibit the release of GABA, a neurotransmitter which prevents the release of dopamine.[6][14] Thus, the inhibition of GABA release by β-endorphin allows for a greater release of dopamine, in part contributing to the analgesic effect of β-endorphin.[6][14] The combination of these pathways reduces pain sensation, allowing for the body to stop a pain impulse once it has been sent.
β-Endorphin has approximately 18 to 33 times the analgesic potency of morphine,[17] though its hormonal effect is species dependent.[7]
Exercise
β-Endorphin release in response to exercise has been known and studied since at least the 1980s.[18] Studies have demonstrated that serum concentrations of endogenous opioids, in particular β-endorphin and β-lipotropin, increase in response to both acute exercise and training.[18] The release of β-endorphin during exercise is associated with a phenomenon colloquially known in popular culture as a runner's high.[19]
Mechanism of action
β-Endorphin acts as an agonist that binds to various types of G protein–coupled receptors(GPCRs), most notably to the mu, delta, and kappa opioid receptors. The receptors are responsible for supra-spinal analgesia.
History
β-Endorphin was discovered in camel pituitary extracts by C.H. Li and David Chung.[20] The primary structure of β-endorphin was unknowingly determined 10 years earlier, when Li and colleagues analyzed the sequence of another neuropeptide produced in the pituitary gland, γ-lipotropin. They noticed that the C-terminus region of this neuropeptide was similar to that of some enkephalins, suggesting that it may have a similar function to these neuropeptides. The C-terminal sequence of γ-lipotropin turned out to be the primary sequence of the β-endorphin.[5]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Sydor A, Brown RY, ed (2009). "Chapter 7: Neuropeptides". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 184, 190, 192. ISBN 9780071481274. "Opioid Peptides
β-Endorphin (also a pituitary hormone) ...
Opioid peptides are encoded by three distinct genes. These precursors include POMC, from which the opioid peptide β-endorphin and several nonopioid peptides are derived, as discussed earlier; proenkephalin, from which met-enkephalin and leu-enkephalin are derived; and prodynorphin, which is the precursor of dynorphin and related peptides. Although they come from different precursors, opioid peptides share significant amino acid sequence identity. Specifically, all of the well-validated endogenous opioids contain the same four N-terminal amino acids (Tyr-Gly-Gly-Phe), followed by either Met or Leu ... Among endogenous opioid peptides, β-endorphin binds preferentially to μ receptors. ... Shared opioid peptide sequences. Although they vary in length from as few as five amino acids (enkephalins) to as many as 31 (β-endorphin), the endogenous opioid peptides shown here contain a shared N-terminal sequence followed by either Met or Leu." - ↑ "Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins". Future Medicinal Chemistry 4 (2): Table 1: Endogenous opioid peptides. February 2012. doi:10.4155/fmc.11.195. PMID 22300099.
- ↑ DBGET
- ↑ 4.0 4.1 4.2 "The effects of beta-endorphin: state change modification". Fluids and Barriers of the CNS 12: 3. January 2015. doi:10.1186/2045-8118-12-3. PMID 25879522.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "60 YEARS OF POMC: Lipotropin and beta-endorphin: a perspective". Journal of Molecular Endocrinology 56 (4): T13-25. May 2016. doi:10.1530/JME-16-0033. PMID 26903509.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Physiology of beta-endorphins. A close-up view and a review of the literature". Biomedicine & Pharmacotherapy 47 (8): 311–20. 1993. doi:10.1016/0753-3322(93)90080-5. PMID 7520295.
- ↑ 7.0 7.1 "beta-Endorphin: analgesic and hormonal effects in humans". Proceedings of the National Academy of Sciences of the United States of America 76 (10): 5377–81. October 1979. doi:10.1073/pnas.76.10.5377. PMID 291954. Bibcode: 1979PNAS...76.5377F.
- ↑ 8.0 8.1 "Opioid receptors: μ receptor". International Union of Basic and Clinical Pharmacology. 15 March 2017. http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319. "Principal endogenous agonists (Human)
β-endorphin (POMC, P01189), [Met]enkephalin (PENK, P01210), [Leu]enkephalin (PENK, P01210) ...
Comments: β-Endorphin is the highest potency endogenous ligand" - ↑ "Allostery at opioid receptors: modulation with small molecule ligands". British Journal of Pharmacology 175 (14): 2846–2856. 2018. doi:10.1111/bph.13823. PMID 28419415.
- ↑ "Molecular mechanisms of opioid receptor-dependent signaling and behavior". Anesthesiology 115 (6): 1363–81. December 2011. doi:10.1097/ALN.0b013e318238bba6. PMID 22020140.
- ↑ "G protein regulation of potassium ion channels". Pharmacological Reviews 50 (4): 723–60. December 1998. PMID 9860808. http://pharmrev.aspetjournals.org/content/50/4/723.
- ↑ "Activation of the Cloned Muscarinic Potassium Channel by G Protein βγ Subunits". Nature 370 (6485): 143–146. July 1994. doi:10.1038/370143a0. PMID 8022483. Bibcode: 1994Natur.370..143R.
- ↑ "The neurobiology of opioid dependence: implications for treatment". Science & Practice Perspectives 1 (1): 13–20. July 2002. doi:10.1151/spp021113. PMID 18567959.
- ↑ 14.0 14.1 14.2 14.3 "Understanding endorphins and their importance in pain management". Hawaii Medical Journal 69 (3): 70–1. March 2010. PMID 20397507.
- ↑ 15.0 15.1 15.2 15.3 "Action of β-endorphin and nonsteroidal anti-inflammatory drugs, and the possible effects of nonsteroidal anti-inflammatory drugs on β-endorphin" (in en). Journal of Clinical Anesthesia 37: 123–128. February 2017. doi:10.1016/j.jclinane.2016.12.016. PMID 28235500.
- ↑ "Opioids and the immune system – friend or foe". British Journal of Pharmacology 175 (14): 2717–2725. 2018. doi:10.1111/bph.13750. PMID 28213891.
- ↑ "beta-endorphin is a potent analgesic agent". Proceedings of the National Academy of Sciences of the United States of America 73 (8): 2895–8. August 1976. doi:10.1073/pnas.73.8.2895. PMID 8780. Bibcode: 1976PNAS...73.2895L.
- ↑ 18.0 18.1 "Endorphins and exercise". Sports Medicine 1 (2): 154–71. March–April 1984. doi:10.2165/00007256-198401020-00004. PMID 6091217.
- ↑ Goldberg, Joseph (19 February 2014). "Exercise and Depression". http://www.webmd.com/depression/guide/exercise-depression.
- ↑ "Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands". Proceedings of the National Academy of Sciences of the United States of America 73 (4): 1145–8. April 1976. doi:10.1073/pnas.73.4.1145. PMID 1063395. Bibcode: 1976PNAS...73.1145L.
External links
- CID 16132316 from PubChem – β-endorphin
- CID 3081525 from PubChem – β-endorphin (1-9)
- CID 133304 from PubChem – β-endorphin (2-9)
- β-endorphin at the US National Library of Medicine Medical Subject Headings (MeSH)
