Biology:Avibactam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Avycaz (formulated with ceftazidime) |
| License data | |
| Pregnancy category |
|
| Routes of administration | IV |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (intravenous) |
| Protein binding | 5.7–8.2%[1] |
| Metabolism | Nil |
| Onset of action | Increases in proportion to dose |
| Excretion | Renal (97%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
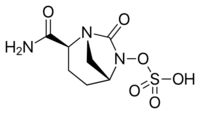
| Formula | C7H11N3O6S |
| Molar mass | 265.24 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Avibactam is a non-β-lactam β-lactamase inhibitor[2] developed by Actavis (now Teva) jointly with AstraZeneca. A new drug application for avibactam in combination with ceftazidime (branded as Avycaz) was approved by the FDA on February 25, 2015, for treating complicated urinary tract (cUTI) and complicated intra-abdominal infections (cIAI) caused by antibiotic resistant-pathogens, including those caused by multi-drug resistant Gram-negative bacterial pathogens.[3][4][5]
Increasing resistance to cephalosporins among Gram-(−) bacterial pathogens, especially among hospital-acquired infections, results in part from the production of β-lactamase enzymes that deactivate these antibiotics. While the co-administration of a β-lactamase inhibitor can restore antibacterial activity to the cephalosporin, previously approved β-lactamase inhibitors such as tazobactam and clavulanic acid do not inhibit important classes of β-lactamases, including Klebsiella pneumoniae carbapenemases (KPCs), New Delhi metallo-β-lactamase 1 (NDM-1), and AmpC-type β-lactamases. Whilst avibactam inhibits class A (KPCs, CTX-M, TEM, SHV), class C (AmpC), and, some, class D serine β-lactamases (such as OXA-23, OXA-48), it has been reported to be a poor substrate/weak inhibitor of class B metallo-β-lactamases, such as VIM-2, VIM-4, SPM-1, BcII, NDM-1, Fez-1.[6]
For infections sustained by metallo-β-lactamases producing bacteria, a therapeutic strategy consists in administering avibactam as companion drug administered alongside aztreonam. In fact, although in theory aztreonam is not hydrolyzed by metallo-β-lactamases, many metallo-β-Lactamases-producing strains co-produce enzymes that could hydrolyze aztreonam (e.g. AmpC, ESBL), therefore avibactam is given to protect aztreonam exploiting its robust β-lactamases inhibition.[7]
See also
References
- ↑ "Full Prescribing Information: AVYCAZ™ (ceftazidime-avibactam) for Injection, for intravenous use". ©2015 Actavis. All rights reserved.. http://pi.actavis.com/data_stream.asp?product_group=1957&p=pi&language=E.
- ↑ "The road to avibactam: the first clinically useful non-β-lactam working somewhat like a β-lactam". Future Medicinal Chemistry 8 (10): 1063–1084. June 2016. doi:10.4155/fmc-2016-0078. PMID 27327972.
- ↑ "Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination". Drugs 73 (2): 159–177. February 2013. doi:10.1007/s40265-013-0013-7. PMID 23371303. https://zenodo.org/record/1230313.
- ↑ "Actavis Announces FDA Acceptance of the NDA Filing for Ceftazidime-Avibactam, a Qualified Infectious Disease Product". Actavis plc. http://www.actavis.com/news/news/thomson-reuters/actavis-announces-fda-acceptance-of-the-nda-filing.
- ↑ "Kinetics of avibactam inhibition against Class A, C, and D β-lactamases". The Journal of Biological Chemistry 288 (39): 27960–27971. September 2013. doi:10.1074/jbc.M113.485979. PMID 23913691.
- ↑ "Interaction of Avibactam with Class B Metallo-β-Lactamases". Antimicrobial Agents and Chemotherapy 60 (10): 5655–5662. October 2016. doi:10.1128/AAC.00897-16. PMID 27401561.
- ↑ "The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases". Antibiotics 10 (8): 1012. August 2021. doi:10.3390/antibiotics10081012. PMID 34439062.
Further reading
- "Pharmacokinetics of avibactam (AVI) and ceftazidime (CAZ) following separate or combined administration in healthy volunteers.". 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). 10. September 2013. p. 13. Poster A-1019. http://www.icaaconline.com/php/icaac2014abstracts/data/papers/2013/A/2013_A-1019.htm.
 |

