Chemistry:CIM-0216
From HandWiki
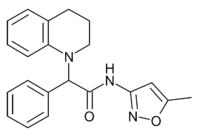
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-(3,4-Dihydroquinolin-1(2H)-yl)-N-(5-methyl-1,2-oxazol-3-yl)-2-phenylacetamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C21H21N3O2 | |
| Molar mass | 347.418 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
CIM-0216 is a chemical compound which acts as a potent and selective activator of the TRPM3 calcium channel. It produces nociception and inflammation and is used to study the function of the TRPM3 receptor in these processes.[1][2][3][4]
References
- ↑ "Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release". Proceedings of the National Academy of Sciences of the United States of America 112 (11): E1363-72. March 2015. doi:10.1073/pnas.1419845112. PMID 25733887.
- ↑ "Transient receptor potential TRPM3 channels: Pharmacology, signaling, and biological functions". Pharmacological Research 124: 92–99. October 2017. doi:10.1016/j.phrs.2017.07.014. PMID 28720517.
- ↑ "Volatile anaesthetics inhibit the thermosensitive nociceptor ion channel transient receptor potential melastatin 3 (TRPM3)". Biochemical Pharmacology 174: 113826. April 2020. doi:10.1016/j.bcp.2020.113826. PMID 31987857.
- ↑ "Functional expression and pharmacological modulation of TRPM3 in human sensory neurons". British Journal of Pharmacology 177 (12): 2683–2695. June 2020. doi:10.1111/bph.14994. PMID 31985045.
 |
