Chemistry:Tibolone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Livial, Tibella, Tibofem, others |
| Other names | TIB; ORG-OD-14; 7α-Methylnoretynodrel; 7α-Methyl-17α-ethynyl-19-nor-δ5(10)-testosterone; 17α-Ethynyl-7α-methylestr-5(10)-en-17β-ol-3-one; 7α-Methyl-19-nor-17α-pregn-5(10)-en-20-yn-17-ol-3-one |
| AHFS/Drugs.com | Professional Drug Facts |
| Pregnancy category |
|
| Routes of administration | By mouth[1] |
| Drug class | Progestogen; Progestin; Estrogen; Androgen; Anabolic steroid |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 92%[5] |
| Protein binding | 96.3% (to albumin; low affinity for SHBG)[5] |
| Metabolism | Liver, intestines (hydroxyl-ation, isomerization, conjugation)[1][8] |
| Metabolites | • Δ4-Tibolone[6] • 3α-Hydroxytibolone[6] • 3β-Hydroxytibolone[6] • Sulfate conjugates[7] |
| Elimination half-life | 45 hours[8] |
| Excretion | Urine: 40%[5] Feces: 60%[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis.[1][9][10][11] The medication is available alone and is not formulated or used in combination with other medications.[12] It is taken by mouth.[1]
Side effects of tibolone include acne and increased hair growth among others.[8] Tibolone is a synthetic steroid with weak estrogenic, progestogenic, and androgenic activity, and hence is an agonist of the estrogen, progesterone, and androgen receptors.[13][1][8][6] It is a prodrug of several metabolites.[1][13][14] The estrogenic effects of tibolone may show tissue selectivity in their distribution.[13][15][14][16]
Tibolone was developed in the 1960s and was introduced for medical use in 1988.[17][18] It is marketed widely throughout the world.[12][19] The medication is not available in the United States.[12][19]
Medical uses
Tibolone is used in the treatment of menopausal symptoms like hot flashes and vaginal atrophy, postmenopausal osteoporosis, and endometriosis.[1][20][11] It has similar or greater effectiveness compared to older menopausal hormone therapy medications, but shares a similar side effect profile.[21][22][23] It has also been investigated as a possible treatment for female sexual dysfunction.[24]
Tibolone reduces hot flashes, prevents bone loss, improves vaginal atrophy and urogenital symptoms (e.g., vaginal dryness, dyspareunia), and has positive effects on mood and sexual function.[25][22][26] The medication may have greater benefits on libido than standard menopausal hormone therapy, which may be related to its androgenic effects.[22][26] It is associated with low rates of vaginal bleeding and breast pain.[25]
A 2015 network meta-analysis of randomized controlled trials found that tibolone was associated with a significantly decreased risk of breast cancer (RR = 0.317).[27] The decrease in risk was greater than that observed with most of the aromatase inhibitors and selective estrogen receptor modulators that were included in the analysis.[27] However, paradoxically, other research has found evidence supporting an increased risk of breast cancer with tibolone.[28][29]
Available forms
Tibolone is available in the form of 2.5 mg oral tablets.[30] It is typically used once daily at a dosage of 1.25 or 2.5 mg.[30]
Side effects
A report in September 2009 from Health and Human Services' Agency for Healthcare Research and Quality suggests that tamoxifen, raloxifene, and tibolone used to reduce the risk of breast cancer significantly reduce the occurrence of invasive breast cancer in midlife and older women, but also increase the risk of adverse effects.[31]
Tibolone can infrequently produce androgenic side effects such as acne and increased facial hair growth.[8] Such side effects have been found to occur in 3 to 6% of treated women.[8]
A 2016 Cochrane review has been published on the short-term and long-term effects of tibolone, including adverse effects.[32] Possible adverse effects of tibolone include unscheduled vaginal bleeding (OR = 2.79; incidence 13–26% more than placebo), an increased risk of breast cancer in women with a history of breast cancer (OR = 1.5) although apparently not without a history of breast cancer (OR = 0.52), an increased risk of cerebrovascular events (strokes) (OR = 1.74) and cardiovascular events (OR = 1.38), and an increased risk of endometrial cancer (OR = 2.04).[32] However, most of these figures are based on very low-quality evidence.[32]
Tibolone has been associated with increased risk of endometrial cancer in most studies.[33]
Pharmacology
Pharmacodynamics
Tibolone possesses a complex pharmacology and has weak estrogenic, progestogenic, and androgenic activity.[8][1][6] Tibolone, 3α-hydroxytibolone, and 3β-hydroxytibolone act as agonists of the estrogen receptors.[1][6] Tibolone and its metabolite δ4-tibolone act as agonists of the progesterone and androgen receptors,[34] while 3α-hydroxytibolone and 3β-hydroxytibolone, conversely, act as antagonists of these receptors.[6] Relative to other progestins, tibolone, including its metabolites, has been described as possessing moderate functional antiestrogenic activity (that is, progestogenic activity), moderate estrogenic activity, high androgenic activity, and no clinically significant glucocorticoid, antiglucocorticoid, mineralocorticoid, or antimineralocorticoid activity.[1][35] The ovulation-inhibiting dosage of tibolone is 2.5 mg/day.[1]
Estrogenic activity
Tibolone and its two major active metabolites, 3α-hydroxytibolone and 3β-hydroxytibolone, act as potent, fully activating agonists of the estrogen receptor (ER), with a high preference for the ERα.[6][34][15] These estrogenic metabolites of tibolone have much weaker activity as estrogens than estradiol (e.g., have 3–29% of the affinity of estradiol for the ER), but occur at relatively high concentrations that are sufficient for full and marked estrogenic responses to occur.[1][15][36]
The estrogenic effects of tibolone show tissue selectivity in their distribution, with desirable effects in bone, the brain, and the vagina, and lack of undesirable action in the uterus, breast, and liver.[15][13][14] The observations of tissue selectivity with tibolone have been theorized to be the result of metabolism, enzyme modulation (e.g., of estrogen sulfatase and estrogen sulfotransferase), and receptor modulation that vary in different target tissues.[34][15] This selectivity differs mechanistically from that of selective estrogen receptor modulators (SERMs) such as tamoxifen, which produce their tissue selectivity via means of modulation of the ER.[34][15] As such, to distinguish it from SERMs, tibolone has been variously described as a "selective tissue estrogenic activity regulator" (STEAR),[15] "selective estrogen enzyme modulator" (SEEM),[16] or "tissue-specific receptor and intracrine mediator" (TRIM).[35] More encompassingly, tibolone has also been described as a "selective progestogen, estrogen, and androgen regulator" (SPEAR), which is meant to reflect the fact that it is tissue-selective and that it regulates effects not only of estrogens but of all three of the major sex hormone classes.[35] Although indications of tissue selectivity with tibolone have been observed, the medication has paradoxically nonetheless been associated with increased risk of endometrial cancer and breast cancer in clinical studies.[32]
It was reported in 2002 that tibolone or its metabolite δ4-tibolone is transformed by aromatase into the potent estrogen 7α-methylethinylestradiol in women, analogously to the transformation of norethisterone into ethinylestradiol.[37] Controversy and disagreement followed when other researchers contested the findings however.[38][39][40][41][42][43] By 2008, these researchers had asserted that tibolone is not aromatized in women and that the previous findings of 7α-methylethinylestradiol detection were merely a methodological artifact.[40][42][43] In accordance, a 2009 study found that an aromatase inhibitor had no effect on the estrogenic potencies of tibolone or its metabolites in vitro, unlike the case of testosterone.[6] In addition, another 2009 study found that the estrogenic effects of tibolone on adiposity in rats do not require aromatization (as indicated by the use of aromatase knockout mice), further in support that 3α-hydroxytibolone and 3β-hydroxytibolone are indeed responsible for such effects.[44] These findings are also in accordance with the fact that tibolone decreases sex hormone-binding globulin (SHBG) levels by 50% in women and does not increase the risk of venous thromboembolism (VTE) (RR = 0.92), which would not be expected if the medication formed a potent, liver metabolism-resistant estrogen similar to ethinylestradiol in important quantities.[1][45] (For comparison, combined oral contraceptives containing ethinylestradiol, due mostly or completely to the estrogen component, have been found to increase SHBG levels by 200 to 400% and to increase the risk of VTE by about 4-fold (OR = 4.03).)[46][47]
In spite of the preceding, others have held, as recently as 2011, that tibolone is converted into 7α-methylethinylestradiol in small quantities.[48][49] They have claimed that 19-nortestosterone derivatives like tibolone, due to lacking a C19 methyl group, indeed are not substrates of the classical aromatase enzyme, but instead are still transformed into the corresponding estrogens by other cytochrome P450 monooxygenases.[41][48][49] In accordance, the closely structurally related AAS trestolone (7α-methyl-19-nortestosterone or 17α-desethynyl-δ4-tibolone) has been found to be transformed into 7α-methylestradiol by human placental microsomes in vitro.[43][50] Also in accordance, considerably disproportionate formation of ethinylestradiol occurs when norethisterone is taken orally (and hence undergoes first-pass metabolism in the liver) relative to parenterally,[51][52] despite the absence of aromatase in the adult human liver.[49][53]
Progestogenic activity
Tibolone and δ4-tibolone act as agonists of the progesterone receptor (PR).[1][49][54] Tibolone has low affinity of 6% of that of promegestone for the PR, while δ4-tibolone has high affinity of 90% of that of promegestone for the PR.[1][49] In spite of its high affinity for the PR however, δ4-tibolone possesses only weak progestogenic activity, about 13% of that of norethisterone.[1][49] The weak progestogenic activity of tibolone may not be sufficient to fully counteract estrogenic activity of tibolone in the uterus and may be responsible for the increased risk of endometrial cancer that has been observed with tibolone in women in large cohort studies.[1][49]
Androgenic activity
Tibolone, mainly via δ4-tibolone, has androgenic activity.[49][1] Whereas tibolone itself has only about 6% of the affinity of metribolone for the androgen receptor, δ4-tibolone has relatively high affinity of about 35% of the affinity of metribolone for this receptor.[49][1] At typical clinical dosages in women, the androgenic effects of tibolone are weak.[49][1] However, relative to other 19-nortestosterone progestins, the androgenic activity of tibolone is high, with a potency comparable to that of testosterone.[49][1] Indeed, the androgenic effects of tibolone have been ranked as stronger than those of all other commonly used 19-nortestosterone progestins (e.g., norethisterone, levonorgestrel, others).[49][1]
The androgenic effects of tibolone have been postulated to be involved in the reduced breast cell proliferation, reduced breast cancer risk, improvement in sexual function, less unfavorable changes in hemostatic parameters relative to estrogen–progestogen combinations, and changes in liver protein synthesis (e.g., 30% reductions in HDL cholesterol levels, 20% reduction in triglyceride levels, and 50% reduction in SHBG levels) observed with tibolone.[49][1] They are also responsible for the androgenic side effects of tibolone such as acne and increased hair growth in some women.[8]
Other activities
Tibolone, 3α-hydroxytibolone, and 3β-hydroxytibolone act as antagonists of the glucocorticoid and mineralocorticoid receptors, with preference for the mineralocorticoid receptor.[6] However, their affinities for these receptors are low, and tibolone has been described as possessing no clinically significant glucocorticoid, antiglucocorticoid, mineralocorticoid, or antimineralocorticoid activity.[1][35]
Pharmacokinetics
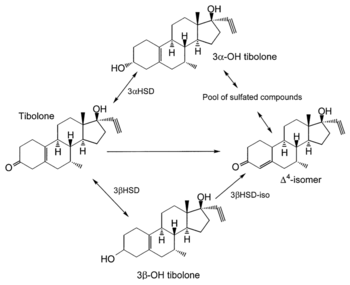
The mean oral bioavailability of tibolone is 92%.[5] Its plasma protein binding is 96.3%.[5] It is bound to albumin, and both tibolone and its metabolites have low affinity for SHBG.[5][1] Tibolone is metabolized in the liver and intestines.[1][8] It is a prodrug and is rapidly transformed into several metabolites, including δ4-tibolone, 3α-hydroxytibolone, and 3β-hydroxytibolone, as well as sulfate conjugates of these metabolites.[1][54][7] 3α-Hydroxytibolone is formed by 3α-hydroxysteroid dehydrogenase, 3β-hydroxytibolone is formed by 3β-hydroxysteroid dehydrogenase, δ4-tibolone is formed by Δ5-4-isomerase, and the sulfate conjugates of tibolone and its metabolites are formed by sulfotransferases, mainly SULT2A1.[35] [55] The sulfate conjugates can be transformed back into free steroids by steroid sulfatase.[56] Following a single oral dose of 2.5 mg tibolone, peak serum levels of tibolone were 1.6 ng/mL, of δ4-tibolone were 0.8 ng/mL, of 3α-hydroxytibolone were 16.7 ng/mL, and of 3β-hydroxytibolone were 3.7 ng/mL after 1 to 2 hours.[1] The elimination half-life of tibolone is 45 hours.[8] It is excreted in urine 40% and feces 60%.[5][8]
Chemistry
Tibolone, also known as 7α-methylnoretynodrel, as well as 7α-methyl-17α-ethynyl-19-nor-δ5(10)-testosterone or as 7α-methyl-17α-ethynylestr-5(10)-en-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone and 19-nortestosterone.[9][1] It is more specifically a derivative of norethisterone (17α-ethynyl-19-nortestosterone) and is a member of the estrane subgroup of the 19-nortestosterone family of progestins.[1][57][58][17] Tibolone is the 7α-methyl derivative of the progestin noretynodrel (17α-ethynyl-δ5(10)-19-nortestosterone).[1] Other steroids related to tibolone include the progestin norgesterone (17α-vinyl-δ5(10)-19-nortestosterone) and the anabolic steroids trestolone (7α-methyl-19-nortestosterone) and mibolerone (7α,17α-dimethyl-19-nortestosterone).[9]
History
Tibolone was developed in the 1960s.[17] It was first introduced in the Netherlands in 1988, and was subsequently introduced in the United Kingdom in 1991.[18][59]
Society and culture
Generic names
Tibolone is the generic name of the drug and its INN, USAN, BAN, DCF, and JAN.[9][10] It is also known by its developmental code name ORG-OD-14.[8]
Brand names
Tibolone is marketed under the brand names Livial, Tibofem, and Ladybon among others.[9][10][12]
Availability
Tibolone is used widely in the European Union, Asia, Australasia, and elsewhere in the world, but is not available in the United States.[12][19][60]
Legal status
Tibolone is a Schedule IV controlled substance in Canada under the 1996 Controlled Drugs and Substances Act.[2][61] It is classified as an anabolic steroid under this act, due to its relatively high activity as an AR agonist, and is the only norethisterone (17α-ethynyl-19-nortestosterone) derivative that is classified as such.[2][61] Tibolone is banned by WADA as an anabolic steroid category S1 largely due to its conversion to the delta-4 tibolone metabolite, which is a potent androgen.[62]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ 2.0 2.1 2.2 "Controlled Drugs and Substances Act (S.C. 1996, c. 19)". 2016-11-30. http://laws-lois.justice.gc.ca/eng/acts/C-38.8/.
- ↑ "Summary Basis of Decision (SBD) for Tibella". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00456&lang=en.
- ↑ "Livial 2.5mg tablets - Summary of Product Characteristics (SmPC)". 29 September 2020. https://www.medicines.org.uk/emc/product/1597.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 "Tibolone 2.5 mg Tablets". Public Assessment Report. United Kingdom Medicines and Healthcare products Regulatory Agency (MHRA). http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con2023754.pdf.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 "Regulation of activities of steroid hormone receptors by tibolone and its primary metabolites". The Journal of Steroid Biochemistry and Molecular Biology 116 (1–2): 8–14. August 2009. doi:10.1016/j.jsbmb.2009.03.008. PMID 19464167. https://www.hal.inserm.fr/inserm-00396319/file/Maudelonde_Table_1.pdf.
- ↑ 7.0 7.1 7.2 "Sulfation of tibolone and tibolone metabolites by expressed human cytosolic sulfotransferases". The Journal of Steroid Biochemistry and Molecular Biology 88 (4–5): 383–391. April 2004. doi:10.1016/j.jsbmb.2004.01.005. PMID 15145448.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 "Tibolone: a review". Maturitas 30 (3): 295–305. November 1998. doi:10.1016/S0378-5122(98)00059-0. PMID 9881330.
- ↑ 9.0 9.1 9.2 9.3 9.4 Dictionary of Pharmacological Agents. CRC Press. 21 November 1996. pp. 1974–. ISBN 978-0-412-46630-4. https://books.google.com/books?id=A0THacd46ZsC&pg=PA1974.
- ↑ 10.0 10.1 10.2 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 275–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA275.
- ↑ 11.0 11.1 "Tibolone". AdisInsight. https://adisinsight.springer.com/drugs/800008188.
- ↑ 12.0 12.1 12.2 12.3 12.4 "Tibolone International". Drugs.com. https://www.drugs.com/international/tibolone.html.
- ↑ 13.0 13.1 13.2 13.3 Menopause: A Comprehensive Approach. Springer. 2 November 2017. pp. 103–. ISBN 978-3-319-59318-0. https://books.google.com/books?id=leM8DwAAQBAJ&pg=PA103.
- ↑ 14.0 14.1 14.2 Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer. 14 June 2017. pp. 182–. ISBN 978-3-319-52210-4. https://books.google.com/books?id=pzgoDwAAQBAJ&pg=PA182.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 Climacteric Medicine - Where Do We Go?: Proceedings of the 4th Workshop of the International Menopause Society. CRC Press. 22 September 2004. pp. 126–. ISBN 978-0-203-02496-6. https://books.google.com/books?id=jaOebeNbAkUC&pg=PA126.
- ↑ 16.0 16.1 Pharmacology for Women's Health. Jones & Bartlett Learning. 25 October 2010. pp. 371–. ISBN 978-0-7637-5329-0. https://books.google.com/books?id=u1wq63x4VsYC&pg=PA371.
- ↑ 17.0 17.1 17.2 Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. 28 March 2012. pp. 769–. ISBN 978-1-4511-4847-3. https://books.google.com/books?id=KZLubBxJEwEC&pg=PA769.
- ↑ 18.0 18.1 "Tibolone and endometrial cancer: a cohort and nested case-control study in the UK". Drug Safety 28 (3): 241–249. 2005. doi:10.2165/00002018-200528030-00005. PMID 15733028.
- ↑ 19.0 19.1 19.2 Hormone Use in Menopause and Male Andropause : A Choice for Women and Men: A Choice for Women and Men. Oxford University Press, USA. 4 October 2003. pp. 73–. ISBN 978-0-19-803620-3. https://archive.org/details/hormoneuseinmeno0000sega.
- ↑ "Hormone therapy for endometriosis and surgical menopause". The Cochrane Database of Systematic Reviews (1): CD005997. January 2009. doi:10.1002/14651858.CD005997.pub2. PMID 19160262.
- ↑ "Tibolone: the way to beat many a postmenopausal ailments". Expert Opinion on Pharmacotherapy 9 (6): 1039–1047. April 2008. doi:10.1517/14656566.9.6.1039. PMID 18377345.
- ↑ 22.0 22.1 22.2 "Role of androgens, progestins and tibolone in the treatment of menopausal symptoms: a review of the clinical evidence". Clinical Interventions in Aging 3 (1): 1–8. 2008. doi:10.2147/CIA.S1043. PMID 18488873.
- ↑ "Hormone therapy for postmenopausal breast cancer survivors: a survey among obstetrician-gynaecologists". European Journal of Gynaecological Oncology 30 (1): 82–84. 2009. PMID 19317264.
- ↑ "Comparative effects of conventional hormone replacement therapy and tibolone on climacteric symptoms and sexual dysfunction in postmenopausal women". Climacteric 13 (2): 147–156. April 2010. doi:10.1080/13697130903009195. PMID 19731119.
- ↑ 25.0 25.1 "Tibolone: clinical recommendations and practical guidelines. A report of the International Tibolone Consensus Group". Maturitas 51 (1): 21–28. May 2005. doi:10.1016/j.maturitas.2005.02.011. PMID 15883105.
- ↑ 26.0 26.1 "The effects of tibolone on mood and libido". Menopause 9 (3): 162–170. 2002. doi:10.1097/00042192-200205000-00004. PMID 11973439.
- ↑ 27.0 27.1 "Breast Cancer Chemoprevention: A Network Meta-Analysis of Randomized Controlled Trials". Journal of the National Cancer Institute 108 (2). February 2016. doi:10.1093/jnci/djv318. PMID 26582062.
- ↑ "Tibolone and breast cancer". Postgraduate Medical Journal 82 (972): 658–662. October 2006. doi:10.1136/pgmj.2005.037184. PMID 17068276.
- ↑ "Effects of tibolone on the breast of postmenopausal women". Taiwanese Journal of Obstetrics & Gynecology 46 (2): 121–126. June 2007. doi:10.1016/S1028-4559(07)60005-9. PMID 17638619.
- ↑ 30.0 30.1 Meeta (15 December 2013). Postmenopausal Osteoporosis: Basic and Clinical Concepts. Jaypee Brothers Publishers. pp. 117–. ISBN 978-93-5090-833-4. https://books.google.com/books?id=72cbBQAAQBAJ&pg=PA117.
- ↑ "Medications Effective in Reducing Risk of Breast Cancer But Increase Risk of Adverse Effects, New Report Says". U.S. Department of Health & Human Services - Agency for Healthcare Research and Quality. September 2009. http://archive.ahrq.gov/news/newsroom/press-releases/2009/brcanmed.html.
- ↑ 32.0 32.1 32.2 32.3 "Short-term and long-term effects of tibolone in postmenopausal women". The Cochrane Database of Systematic Reviews 10 (10): CD008536. October 2016. doi:10.1002/14651858.CD008536.pub3. PMID 27733017.
- ↑ "Hormone replacement therapy and the risk of endometrial cancer: A systematic review". Maturitas 91: 25–35. September 2016. doi:10.1016/j.maturitas.2016.05.013. PMID 27451318.
- ↑ 34.0 34.1 34.2 34.3 Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer Science & Business Media. 22 May 2013. pp. 152–. ISBN 978-1-4614-6837-0. https://books.google.com/books?id=TAYnR1b8jRkC&pg=PA152.
- ↑ 35.0 35.1 35.2 35.3 35.4 "What is tibolone--and is it a SPEAR?". Climacteric 5 (3): 236–239. September 2002. doi:10.1080/cmt.5.3.236.239. PMID 12419081.
- ↑ "Hormonal properties of norethisterone, 7alpha-methyl-norethisterone and their derivatives". The Journal of Steroid Biochemistry and Molecular Biology 74 (4): 213–222. November 2000. doi:10.1016/s0960-0760(00)00125-4. PMID 11162927.
- ↑ "Formation of 7 alpha-methyl-ethinyl estradiol during treatment with tibolone". Menopause 9 (4): 293–295. 2002. doi:10.1097/00042192-200207000-00011. PMID 12082366.
- ↑ "Tibolone is not converted by human aromatase to 7alpha-methyl-17alpha-ethynylestradiol (7alpha-MEE): analyses with sensitive bioassays for estrogens and androgens and with LC-MSMS". Steroids 68 (3): 235–243. March 2003. doi:10.1016/S0039-128X(02)00184-8. PMID 12628686.
- ↑ "Lack of aromatisation of the 3-keto-4-ene metabolite of tibolone to an estrogenic derivative". Steroids 71 (7): 639–646. July 2006. doi:10.1016/j.steroids.2006.03.006. PMID 16712888.
- ↑ 40.0 40.1 "Conversion of tibolone to 7alpha-methyl-ethinyl estradiol using gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry: interpretation and clinical implications". Menopause 13 (6): 926–934. 2006. doi:10.1097/01.gme.0000227331.49081.d7. PMID 17006378.
- ↑ 41.0 41.1 "Can 19-nortestosterone derivatives be aromatized in the liver of adult humans? Are there clinical implications?". Climacteric 10 (4): 344–353. August 2007. doi:10.1080/13697130701380434. PMID 17653961.
- ↑ 42.0 42.1 "7alpha-Methyl-ethinyl estradiol is not a metabolite of tibolone but a chemical stress artifact". Menopause 14 (3 Pt 1): 474–480. 2007. doi:10.1097/01.gme.0000247015.63877.d4. PMID 17237734.
- ↑ 43.0 43.1 43.2 "Tibolone is not aromatized in postmenopausal women". Climacteric 11 (2): 175; author reply 175-175; author reply 176. April 2008. doi:10.1080/13697130701752087. PMID 18365860.
- ↑ "The estrogenic component of tibolone reduces adiposity in female aromatase knockout mice". Menopause 16 (3): 582–588. 2009. doi:10.1097/gme.0b013e31818fb20b. PMID 19182696.
- ↑ "Hormone replacement therapy and the risk of venous thromboembolism: a population-based study". Journal of Thrombosis and Haemostasis 8 (5): 979–986. May 2010. doi:10.1111/j.1538-7836.2010.03839.x. PMID 20230416.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 157–. ISBN 978-92-832-1291-1. https://books.google.com/books?id=aGDU5xibtNgC&pg=PA157.
- ↑ "The epidemiology of venous thromboembolism". Journal of Thrombosis and Thrombolysis 41 (1): 3–14. January 2016. doi:10.1007/s11239-015-1311-6. PMID 26780736.
- ↑ 48.0 48.1 "In vivo conversion of TIB to MEE not an artifact generated by heat". Menopause 14 (2): 331–4; author reply 334–5. 2007. doi:10.1097/01.gme.0000264447.18842.da. PMID 17496790.
- ↑ 49.00 49.01 49.02 49.03 49.04 49.05 49.06 49.07 49.08 49.09 49.10 49.11 49.12 "Pharmacology of progestogens". Journal für Reproduktionsmedizin und Endokrinologie 8 (Special Issue 1): 157–176. 2011. http://www.kup.at/kup/pdf/10168.pdf.
- ↑ "Aromatization of 7 alpha-methyl-19-nortestosterone by human placental microsomes in vitro". The Journal of Steroid Biochemistry and Molecular Biology 48 (2–3): 297–304. February 1994. doi:10.1016/0960-0760(94)90160-0. PMID 8142308.
- ↑ "In vivo conversion of norethisterone and norethisterone acetate to ethinyl etradiol in postmenopausal women". Contraception 56 (6): 379–385. December 1997. doi:10.1016/S0010-7824(97)00174-1. PMID 9494772.
- ↑ "In Vivo Formation of Ethinylestradiol After Intramuscular Administration of Norethisterone Enantate". Journal of Clinical Pharmacology 58 (6): 781–789. June 2018. doi:10.1002/jcph.1079. PMID 29522253.
- ↑ "Aromatase in human liver and its diseases". Cancer Medicine 2 (3): 305–315. June 2013. doi:10.1002/cam4.85. PMID 23930207.
- ↑ 54.0 54.1 "The in vivo metabolism of tibolone in animal species". European Journal of Drug Metabolism and Pharmacokinetics 27 (1): 1–10. 2002. doi:10.1007/BF03190399. PMID 11996321.
- ↑ "Sulfation of tibolone metabolites by human postmenopausal liver and small intestinal sulfotransferases (SULTs)". Steroids 71 (5): 343–351. May 2006. doi:10.1016/j.steroids.2005.11.003. PMID 16360722. http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1083&context=veterans.
- ↑ "Interactions of the human cytosolic sulfotransferases and steroid sulfatase in the metabolism of tibolone and raloxifene". The Journal of Steroid Biochemistry and Molecular Biology 107 (3–5): 202–210. 2007. doi:10.1016/j.jsbmb.2007.03.046. PMID 17662596.
- ↑ Breast Cancer: Prognosis, Treatment, and Prevention. CRC Press. 17 July 2002. pp. 222–. ISBN 978-0-203-90924-9. https://books.google.com/books?id=l4XLBQAAQBAJ&pg=PA222.
- ↑ Trends in Breast Cancer Research. Nova Publishers. 2005. pp. 58–. ISBN 978-1-59454-134-6. https://books.google.com/books?id=vXvljojPp-UC&pg=PA58.
- ↑ "Tibolone and its effects on bone: a review". Climacteric 4 (2): 120–136. June 2001. doi:10.1080/cmt.4.2.120.136. PMID 11428176.
- ↑ Women's Sexual Function and Dysfunction: Study, Diagnosis and Treatment. CRC Press. 17 November 2005. pp. 556–. ISBN 978-1-84214-263-9. https://books.google.com/books?id=3J7TnwpbZQwC&pg=PA556.
- ↑ 61.0 61.1 "Controlled Drugs and Substances Act SCHEDULE IV (Sections 2, 4 to 7.1, 10, 29, 55 and 60)". 2020-10-29. https://laws-lois.justice.gc.ca/eng/acts/C-38.8/section-sched95660.html.
- ↑ "2022 Prohibited List: SUBSTANCES AND METHODS PROHIBITED AT ALL TIMES". WADA. https://www.wada-ama.org/sites/default/files/2022-01/2022list_draft_explanatory_list_en_0.pdf.
Further reading
- "Tibolone (Livial)--a new steroid for the menopause". Drug and Therapeutics Bulletin 29 (20): 77–78. September 1991. PMID 1935591.
- "Tibolone and climacteric symptoms". Maturitas 21 (2): 127–136. February 1995. doi:10.1016/0378-5122(94)00888-E. PMID 7752950.
- "The effects of tibolone". Gynecological Endocrinology 12 (3): 213–220. June 1998. doi:10.3109/09513599809015548. PMID 9675570.
- "Tibolone and the serum lipid/lipoprotein profile: does this have a role in cardiovascular protection in postmenopausal women?". Menopause 6 (2): 87–89. 1999. doi:10.1097/00042192-199906020-00002. PMID 10374212.
- "[Action of SERM and SAS (tibolone) on breast tissue]" (in fr). Contraception, Fertilité, Sexualité 27 (5): 368–375. May 1999. PMID 10401183.
- "[Anti-estrogens, selective estrogen receptor modulators (SERM), tibolone: modes of action]" (in fr). Contraception, Fertilite, Sexualite 27 (9): 620–624. September 1999. PMID 10540506.
- "[Alternatives to hormone replacement therapy: raloxifene and tibolone]" (in de). Zeitschrift für Ärztliche Fortbildung und Qualitätssicherung 94 (3): 205–209. April 2000. PMID 10802895.
- "Tibolone: what does tissue specific activity mean?". Maturitas 37 (3): 159–165. January 2001. doi:10.1016/S0378-5122(00)00184-5. PMID 11173177.
- "Tibolone: a steroid with a tissue-specific mode of action". The Journal of Steroid Biochemistry and Molecular Biology 76 (1–5): 231–238. 2001. doi:10.1016/S0960-0760(01)00044-9. PMID 11384882.
- "Tibolone: new type of hormone replacement". Harvard Women's Health Watch 9 (5): 5. December 2001. PMID 11751099.
- "Tibolone for postmenopausal women: systematic review of randomized trials". The Journal of Clinical Endocrinology and Metabolism 87 (1): 16–23. January 2002. doi:10.1210/jcem.87.1.8141. PMID 11788614.
- "[Drugs in development for the treatment of osteoporosis: Tibolone]" (in ja). Nihon Rinsho. Japanese Journal of Clinical Medicine 60 (Suppl 3): 552–571. March 2002. PMID 11979954.
- "[Tibolone]" (in fr). Presse Médicale 31 (28): 1314–1322. September 2002. PMID 12355994.
- "[Postmenopausal hormonal treatment: conventional hormone replacement therapy or tibolone? Effects on bone]" (in fr). Journal de Gynécologie, Obstétrique et Biologie de la Reproduction 31 (6): 541–549. October 2002. PMID 12407324.
- "Pros and cons of existing treatment modalities in osteoporosis: a comparison between tibolone, SERMs and estrogen (+/-progestogen) treatments". The Journal of Steroid Biochemistry and Molecular Biology 83 (1–5): 157–165. December 2002. doi:10.1016/S0960-0760(03)00055-4. PMID 12650712.
- "Receptor profiling and endocrine interactions of tibolone". Steroids 68 (1): 21–30. January 2003. doi:10.1016/S0039-128X(02)00112-5. PMID 12475720.
- "Tibolone: a unique version of hormone replacement therapy". The Annals of Pharmacotherapy 38 (5): 874–881. May 2004. doi:10.1345/aph.1D462. PMID 15026563.
- "[Tibolone]" (in ja). Nihon Rinsho. Japanese Journal of Clinical Medicine 62 (Suppl 2): 555–559. February 2004. PMID 15035189.
- "A review of the effects of tibolone on the skeleton". Expert Opinion on Pharmacotherapy 5 (4): 941–949. April 2004. doi:10.1517/14656566.5.4.941. PMID 15102576.
- "Tibolone: a selective tissue estrogenic activity regulator (STEAR)". Maturitas 48 (Suppl 1): S4–S6. August 2004. doi:10.1016/j.maturitas.2004.02.013. PMID 15337241.
- "Tissue-selectivity: the mechanism of action of tibolone". Maturitas 48 (Suppl 1): S30–S40. August 2004. doi:10.1016/j.maturitas.2004.02.012. PMID 15337246.
- "Tissue-selective effects of tibolone on the breast". Maturitas 49 (1): S5–S15. September 2004. doi:10.1016/j.maturitas.2004.06.022. PMID 15351102.
- "The effects of tibolone and oestrogen-based HT on breast cell proliferation and mammographic density". Maturitas 49 (1): S16–S21. September 2004. doi:10.1016/j.maturitas.2004.06.011. PMID 15351103.
- "Therapeutic effects of progestins, androgens, and tibolone for menopausal symptoms". The American Journal of Medicine 118 (12): 88–92. December 2005. doi:10.1016/j.amjmed.2005.09.040. PMID 16414332.
- "Metabolism of exogenous sex steroids and effect on brain functions with a focus on tibolone". The Journal of Steroid Biochemistry and Molecular Biology 102 (1–5): 195–204. December 2006. doi:10.1016/j.jsbmb.2006.09.037. PMID 17113982.
- "Tibolone for prevention and treatment of postmenopausal osteoporosis". Maturitas 57 (1): 35–38. May 2007. doi:10.1016/j.maturitas.2007.02.008. PMID 17350774.
- "Postmenopausal tibolone therapy: biologic principles and applied clinical practice". MedGenMed 9 (1): 2. January 2007. PMID 17435612.
- "Postmenopausal HRT and tibolone in relation to muscle strength and body composition". Maturitas 58 (1): 7–18. September 2007. doi:10.1016/j.maturitas.2007.04.012. PMID 17576043.
- "Cardiovascular effects of tibolone: a selective tissue estrogenic activity regulator". Cardiovascular Drug Reviews 25 (2): 132–145. 2007. doi:10.1111/j.1527-3466.2007.00007.x. PMID 17614936.
- "[Relation between hormonal therapy and tibolone with SERMs in postmenopausal women's myomes growth]" (in es). Ginecologia y Obstetricia de Mexico 76 (10): 610–614. October 2008. PMID 19062511.
- "Updated clinical recommendations for the use of tibolone in Asian women". Climacteric 13 (4): 317–327. August 2010. doi:10.3109/13697131003681458. PMID 20443720.
- "Tibolone in postmenopausal women: a review based on recent randomised controlled clinical trials". Gynecological Endocrinology 26 (11): 804–814. November 2010. doi:10.3109/09513590.2010.495437. PMID 20586550.
- "Tibolone decreases Lipoprotein(a) levels in postmenopausal women: A systematic review and meta-analysis of 12 studies with 1009 patients". Atherosclerosis 242 (1): 87–96. September 2015. doi:10.1016/j.atherosclerosis.2015.06.056. PMID 26186655. https://research-repository.uwa.edu.au/en/publications/6e9e0c05-f025-44b7-bab1-a9d49916878d.
- "Effects of Tibolone on the Central Nervous System: Clinical and Experimental Approaches". BioMed Research International 2017: 8630764. 2017. doi:10.1155/2017/8630764. PMID 28191467.
- "The effect of hormone replacement therapy and tibolone on lipoprotein (a) concentrations in postmenopausal women: A systematic review and meta-analysis". Maturitas 99: 27–36. May 2017. doi:10.1016/j.maturitas.2017.02.009. PMID 28364865.
- "Tibolone and risk of gynecological hormone sensitive cancer". International Journal of Cancer 142 (12): 2435–2440. June 2018. doi:10.1002/ijc.31267. PMID 29349823.
External links
- "Tibolone". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tibolone.
 |
