Chemistry:Lanthanum(III) iodide
| |
| Names | |
|---|---|
| Other names
Lanthanum triiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| LaI3 | |
| Molar mass | 519.62 |
| Density | 5.63 g/mL at 25 °C |
| Melting point | 772 °C (1,422 °F; 1,045 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI3.[1]
Synthesis
Lanthanum(III) iodide can be synthesised by the reaction of lanthanum metal with mercury(II) iodide:[2][3]
- 2 La + 3 HgI2 → 2 LaI3 + 3 Hg
It can also be prepared from the elements, that is by the reaction of metallic lanthanum with iodine:[2]
- 2 La + 3 I2 → 2 LaI3
While lanthanum(III) iodide solutions can be generated by dissolving lanthanum oxide in hydroiodic acid, the product will hydrolyse and form polymeric hydroxy species:[4]
- La2O3 + 6 HI → 2 LaI3 + 3 H2O → further reactions
Structure
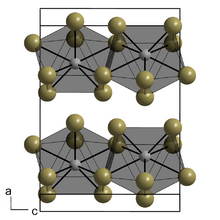
Lanthanum(III) iodide adopts the same crystal structure as plutonium(III) bromide, with 8-coordinate metal centres arranged in layers.[4][5] This orthorhombic structure is typical of the triiodides of the lighter lanthanides (La–Nd), whereas heavier lanthanides tend to adopt the hexagonal bismuth(III) iodide structure.[3]
Reactivity and applications
Lanthanum(III) iodide is very soluble in water and is deliquescent.[4] Anhydrous lanthanum(III) iodide reacts with tetrahydrofuran to form a photoluminescent complex, LaI3(THF)4, with an average La–I bond length of 3.16 Å.[6][7] This complex is a starting material for amide and cyclopentadienyl complexes of lanthanum.[6][8]
Related compounds
Lanthanum also forms a diiodide, LaI2. It is an electride and is best formulated {LaIII,2I−,e−}, with the electron delocalised in a conduction band.[4] Several other lanthanides form similar compounds, including CeI2, PrI2 and GdI2.[9] Lanthanum diiodide adopts the same tetragonal crystal structure as PrI2.[10]
Lanthanum(III) iodide reacts with lanthanum metal under an argon atmosphere in a tantalum capsule at 1225 K to form the mixed-valence compound La2I5.[11]
Reduction of LaI2 or LaI3 with metallic sodium in an argon atmosphere at 550 °C gives lanthanum monoiodide, LaI, which has a hexagonal crystal structure.[12]
References
- ↑ Taylor, Moddie D. (1962). "Preparation of Anhydrous Lanthanon Halides". Chem. Rev. 62 (6): 503–511. doi:10.1021/cr60220a001.
- ↑ 2.0 2.1 Corbett, John D.; Simon, Arndt (1984). "Chapter 6: Lanthanum Triiodide (and Other Rare Earth Metal Triiodides)". in Holt Jr., Smith L.. Inorg. Synth.. 22. pp. 11–16. doi:10.1002/9780470132531.ch6.
- ↑ 3.0 3.1 Asprey, L. B.; Keenan, T. K.; Kruse, F. H. (1964). "Preparation and Crystal Data for Lanthanide and Actinide Triiodides". Inorg. Chem. 3 (8): 1137–1141. doi:10.1021/ic50018a015. https://digital.library.unt.edu/ark:/67531/metadc867868/.
- ↑ 4.0 4.1 4.2 4.3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 949–950. ISBN 978-0-08-037941-8.
- ↑ Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. p. 420. ISBN 978-0-19-965763-6.
- ↑ 6.0 6.1 Ortu, Fabrizio (2022). "Rare Earth Starting Materials and Methodologies for Synthetic Chemistry". Chem. Rev. 122 (6): 6040–6116. doi:10.1021/acs.chemrev.1c00842. PMID 35099940.
- ↑ Li, Yangjuan; Chen, Xiuting; Gong, Yu (2021). "Photoluminescence of LaI3 switched on and off by association and dissociation of non-luminescent tetrahydrofuran". Dalton Trans. 50 (11): 3797–3800. doi:10.1039/D1DT00162K. PMID 33720234.
- ↑ Windorff, Cory J.; Dumas, Megan T.; Ziller, Joseph W.; Gaunt, Andrew J.; Kozimor, Stosh A.; Evans, William J. (2017). "Small-Scale Metal-Based Syntheses of Lanthanide Iodide, Amide, and Cyclopentadienyl Complexes as Analogues for Transuranic Reactions". Inorg. Chem. 56 (19): 11981–11989. doi:10.1021/acs.inorgchem.7b01968. PMID 28915015.
- ↑ Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. p. 1250. ISBN 978-0-19-965763-6.
- ↑ Burrow, J. H.; Maule, C. H.; Strange, P.; Tothill, J. N.; Wilson, J. A. (1987). "The electronic conditions in the 5d1 layer-metal LaI2 making comparison with the iso-electronic tantalum dichalcogenides, with the other RE di-iodides, and with the RE monochalcogenides". J. Phys. C: Solid State Phys. 20 (26): 4115–4133. doi:10.1088/0022-3719/20/26/014. Bibcode: 1987JPhC...20.4115B.
- ↑ Mattausch, Hj.; Oeckler, O.; Simon, A. (2003). "Crystal structure of dilanthanum pentaiodide, La2I5". Z. Kristallogr. NCS 218 (3): 281. doi:10.1524/ncrs.2003.218.3.281.
- ↑ Ryazanov, Mikhail; Kienle, Lorenz; Simon, Arndt; Mattausch, Hansjürgen (2006). "New Synthesis Route to and Physical Properties of Lanthanum Monoiodide". Inorg. Chem. 45 (5): 2068–2074. doi:10.1021/ic051834r. PMID 16499368.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

