Chemistry:Nepafenac
 | |
| Clinical data | |
|---|---|
| Trade names | Nevanac, Ilevro, Amnac, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606007 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
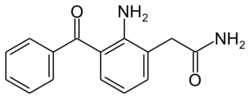
| Formula | C15H14N2O2 |
| Molar mass | 254.289 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nepafenac, sold under the brand name Nevanac among others, is a nonsteroidal anti-inflammatory drug (NSAID), usually sold as a prescription eye drop 0.1% solution (Nevanac) or 0.3% solution (Ilevro). It is used to treat pain and inflammation associated with cataract surgery.[3] Nepafenac is a prodrug of amfenac, an inhibitor of COX-1 and COX-2 activity.[4][5]
Medical uses
Nepafenac is indicated for use in the treatment of pain and inflammation following cataract surgery.[3][6][7][8]
In the European Union nepafenac is also indicated for the reduction in the risk of postoperative macular edema associated with cataract surgery in people with diabetes.[8]
Pharmacology
Mechanism of action
Nepafenac is an NSAID, thought to be a prodrug of amfenac after conversion by ocular tissue hydrolases after penetration via the cornea.[6][7] Amfenac, like other NSAIDs, is thought to inhibit cyclooxygenase action.[6][7]
Adverse events
Side effects include headache; runny nose; pain or pressure in the face; nausea; vomiting; and dry, itchy, sticky eyes.[9] Serious side effects include red or bloody eyes; foreign body sensation in the eye; sensitivity to light; decreased visual acuity; seeing specks or spots; teary eyes; or eye discharge or crusting.[9]
Regulatory
Nevanac
On February 25, 2005, Alcon filed a New Drug Application (NDA) with the U.S. Food and Drug Administration (FDA) for Nevanac 0.1%.[10] Results from the two trials referenced in the NDA (Phase 2/3 study C-02-53; Phase 3 study C-03-32) have not been published.[11] Study C-02-53 consisted of 228 patients across 10 centers in the United States.[12] Study C-03-32 consisted of 522 patients across 22 centers in the United States.[12] The efficacy results presented were confirmed in a study published in 2007.[13]
Nevanac was approved by the FDA on August 19, 2005, with application number 021–862.[14]
Ilevro
An NDA for Ilevro was filed on December 15, 2011.[15] In a one-month study, no new toxicities arose in the new formulation of nepafenac.[16] Safety and efficacy information was derived from the previous Nevanac application.[16] In June 2010, a confirmatory study began (Study C09055) consisting of over 2000 patients from 49 US sites and 37 European sites.[17][18] A second phase 3 trial (Study C11003) was conducted in a population of 1,342 patients at 37 sites across the United States which failed to demonstrate superiority over Nevanac in an altered dosing regimen.[17]
Ilevro was approved by the FDA on October 16, 2012, with application number 203–491.[19]
Commercialization
Both Nevanac and Ilevro are manufactured and sold by Alcon, Inc.[6][7] Alcon is currently a division of Novartis International AG, which is primarily based out of Switzerland.[20] Alcon, Inc. also holds locations in both Switzerland and the United States.[21] The company has gone through several name changes, from Alcon Laboratories, Inc. to Alcon Universal, Ltd., to Alcon, Inc.[21]
Nevanac entered the market in 2005 as a product of Alcon, at the time a subsidiary of Nestlé.[22] On April 6, 2008, Novartis agreed to purchase approximately 74 million shares of Alcon from Nestlé at $143.18 per share.[22] On January 4, 2010, Novartis agreed to purchase all remaining shares of Alcon from Nestlé, totalling 156 million shares or 77% of the shares in the company.[22] At the time of the purchase, a proposal for a merger under Swiss merger law was given to the Alcon board of directors.[22] The merger was agreed upon on December 15, 2010, making Alcon "the second largest division within Novartis."[22] The merger was completed on April 8, 2011.[23]
Ilevro was launched by Alcon on January 21, 2013.[24] In 2014 and 2015, net sales by Alcon grew, contributed to in part by the increased volume in sales of Ilevro.[25][26][27] That financial year, Novartis reported $18 billion in total financial debt.[25] That figure has grown steadily since. In 2016, Novartis reported a total debt of $23.8 billion,[28] up from the $21.9 billion reported in 2015 [27] and the $20.4 billion reported in 2014.[26] As of May 2017, Novartis is estimated to be worth $193.2 billion.[29]
On January 27, 2016, Alcon was moved to become a branch of the Innovative Medicines Division at Novartis.[28] Early in 2016, Alcon formed agreements with both TrueVision and PowerVision, and acquired Transcend Medical.[28] As of January 2017, Novartis is weighing options for Alcon in the business structure.[28]
Commercial risks
Alcon faced declining growth in 2016, having faced challenges in development and marketing of new products.[28]
Marketing
Novartis maintains a detailing unit geared toward health professionals consisting of over 3,000 employees within the United States and an additional 21,000 worldwide.[28] Novartis is also seeking to expand direct-to-consumer advertising and entrance into specialty product markets.[28] Novartis also notes the influence of position and preference on US Centers for Medicare & Medicaid formularies in expanding their market value.[28]
Nepafenac, Nevanac, and Ilevro are all absent from the 2016 Annual Report issued from Novartis.[28]
Intellectual property
There are currently[when?] seven U.S. patents filed that are directly associated with the modernized formulations of nepafenac, all stemming from Novartis.[30] There are three patents associated with Nevanac that are still[when?] active[31] and four associated with Ilevro.[32] The earliest patent related to the modern formulations of nepafenac was approved on June 11, 2002, after being filed in 1999, by Bahram Asgharian.[33] A patent was filed by Warren Wong, associated with Alcon, Inc. based out of Fort Worth, Texas, on December 2, 2005, for aqueous suspensions of nepafenac.[34] Another patent for a nepafenac-based drug was filed on May 8, 2006, by Geoffrey Owen, Amy Brooks, and Gustav Graff.[35] A patent was filed by Masood A. Chowhan and Huagang Chen on February 9, 2007, and approved on May 24, 2011,[36] followed closely by a patent filed by Warren Wong on September 23, 2010, and approved on December 6, 2011.[37] Masood A. Chowhan, Malay Ghosh, Bahram Asgharian, and Wesley Wehsin Han filed another patent on December 1, 2010, and approved on December 30, 2014.[38] The most recent[when?] patent was filed by Masood A. Chowhan, Malay Ghosh, Bahram Asgharian, and Wesley Weshin Han on November 12, 2014, and approved on May 30, 2017.[39] These patents are in effect until dates ranging between July 17, 2018, and March 31, 2032.[32]
Novartis also maintains patents on nepafenac in 26 countries outside the United States.[40]
References
- ↑ "Nepafenac ophthalmic Use During Pregnancy". 6 June 2019. https://www.drugs.com/pregnancy/nepafenac-ophthalmic.html.
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2015". 21 June 2022. https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2015.
- ↑ 3.0 3.1 Nepafenac Monograph
- ↑ Drugbank: Nepafenac
- ↑ "Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: a randomized trial". Indian Journal of Ophthalmology 60 (4): 277–81. July 2012. doi:10.4103/0301-4738.98705. PMID 22824596.
- ↑ 6.0 6.1 6.2 6.3 "Nevanac- nepafenac suspension/ drops". 9 September 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3ad48650-75e3-4c74-9302-01b0088f164e.
- ↑ 7.0 7.1 7.2 7.3 "Ilevro- nepafenac suspension". 9 September 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6c212466-ff8d-ecfc-ede2-ef8bdcaaf114.
- ↑ 8.0 8.1 "Nevanac EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/nevanac.
- ↑ 9.0 9.1 "Nepafenac Ophthalmic". U.S. National Library of Medicine. https://medlineplus.gov/druginfo/meds/a606007.html.
- ↑ "Nevanac Approval Package". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021862s000_Nevanac_approv.pdf.
- ↑ "Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension". Clinical Ophthalmology 2 (2): 355–68. June 2008. doi:10.2147/opth.s1067. PMID 19668727.
- ↑ 12.0 12.1 "Nevanac Statistical Review". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021862s000_Nevanac_statr.pdf.
- ↑ "Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery". Journal of Cataract and Refractive Surgery 33 (1): 53–8. January 2007. doi:10.1016/j.jcrs.2006.08.043. PMID 17189793.
- ↑ "Drug Approval Package: Nevanac (Nepafenac) Ophthalmic Suspension NDA #021862". January 6, 2005. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021862s000_NevanacTOC.cfm.
- ↑ "Ilevro Approval Package". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203491Orig1s000Approv.pdf.
- ↑ 16.0 16.1 "203491 Pharmacology Review". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203491Orig1s000PharmR.pdf.
- ↑ 17.0 17.1 "Ilevro Statistical Review". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203491Orig1s000StatR.pdf.
- ↑ Confirmatory Study Nepafenac 0.3%. U.S. National Library of Medicine. 29 November 2012. https://clinicaltrials.gov/ct2/show/NCT01109173. Retrieved October 31, 2017.
- ↑ "Drug Approval Package: Nepafenac Ophthalmic Suspension, 0.3% NDA #203491". April 8, 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203491_nepafenac_toc.cfm.
- ↑ "About Us". https://www.novartis.com/about-us.
- ↑ 21.0 21.1 "Nevanac Administrative Documents and Correspondence". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021862s000_Nevanac_admincorres.pdf.
- ↑ 22.0 22.1 22.2 22.3 22.4 "Alcon Annual Report 2010". United States Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/1167379/000116737911000037/acl20f2010.pdf.
- ↑ "Alcon Form 15". United States Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/1167379/000110465911021611/a11-7954_41512b.htm.
- ↑ "Alcon Launches ILEVRO™ (nepafenac ophthalmic suspension) 0.3%, a New Non-Steroidal Anti-Inflammatory Drug, for the Treatment of Pain and Inflammation Associated with Cataract Surgery". https://www.alcon.com/news/media-releases/alcon-launches-ilevro-nepafenac-ophthalmic-suspension-03-new-non-steroidal-anti.
- ↑ 25.0 25.1 "Form 20-F". United States Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/1114448/000104746914000415/a2217883z20-f.htm.
- ↑ 26.0 26.1 "Form 20-F". United States Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/1114448/000104746915000433/a2222787z20-f.htm.
- ↑ 27.0 27.1 "Form 20F". United States Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/1114448/000104746916009872/a2227040z20-f.htm.
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 28.6 28.7 28.8 "Form 20-F". United States Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/1114448/000104746917000338/a2230622z20-f.htm.
- ↑ "Novartis on the Forbes Top Multinational Performers List". https://www.forbes.com/companies/novartis/.
- ↑ "Nepafenac". PharmaCompass. https://www.pharmacompass.com/patent-expiry-expiration/nepafenac.
- ↑ "Generic Nevanac Availability". https://www.drugs.com/availability/generic-nevanac.html.
- ↑ 32.0 32.1 "Generic Ilevro Availability". https://www.drugs.com/availability/generic-ilevro.html.
- ↑ "United States Patent Application: 6403609". http://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/search-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN/6403609.
- ↑ "United States Patent Application: 0060122277". http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=/netahtml/PTO/srchnum.html&r=1&f=G&l=50&s1=20060122277.PGNR..
- ↑ "United States Patent Application: 0060257487". http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=/netahtml/PTO/srchnum.html&r=1&f=G&l=50&s1=20060257487.PGNR..
- ↑ "United States Patent: 7947295". http://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/search-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN/7947295.
- ↑ "United States Patent: 8071648". http://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/search-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN/8071648.
- ↑ "United States Patent: 8921337". http://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/search-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN/8921337.
- ↑ "United States Patent: 9662398". http://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/search-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN/9662398.
- ↑ "Nepafenac - Generic Drug Details". thinkBiotech LLC. https://www.drugpatentwatch.com/p/generic-api/nepafenac.
External links
- "Nepafenac". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/nepafenac.
 |
