Chemistry:Niobium(IV) iodide
From HandWiki
| |
| Names | |
|---|---|
| Other names
Niobium tetraiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| I4Nb | |
| Molar mass | 600.52425 g·mol−1 |
| Appearance | grey solid[1] |
| Density | 5.6 g·cm−3[1] |
| Melting point | 503 °C[1] |
| reacts[1] | |
| Related compounds | |
Other anions
|
NbF4, NbCl4, NbBr4 |
Other cations
|
TaI4 |
Related compounds
|
NbI3, NbI5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Niobium(IV) iodide is an iodide of niobium, with the chemical formula of NbI4.
Preparation
Niobium(IV) iodide can be obtained by the decomposition of niobium(V) iodide under a vacuum at 206-270 °C.[2]
Properties
Niobium(IV) iodide is a grey solid that reacts with water.[1]
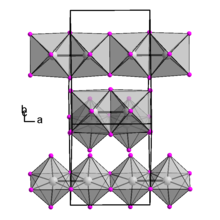
Niobium(IV) iodide is an orthorhombic crystal with space group Cmc21 (No. 36).[3] Its crystal is formed by NbI6 octahedra connected by edges and also contains Nb-Nb bonds. At 348 to 417 °C, the crystal structure of niobium(IV) iodide changes.[4] Niobium(IV) iodide turns into a metal under extremely high pressure.[5]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Haynes, William M.; Lide, David R.; Bruno, Thomas J. (2017). CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data.. Boca Raton, Florida. p. 76. ISBN 978-1-4987-5429-3. OCLC 957751024.
- ↑ Perry, Dale L. (2011). Handbook of Inorganic Compounds. Boca Raton, FL. p. 298. ISBN 978-1-4398-1462-8. OCLC 759865801.
- ↑ Dahl, L. F.; Wampler, D. L. (1962-09-01). "The crystal structure of α-niobium tetraiodide". Acta Crystallographica (International Union of Crystallography (IUCr)) 15 (9): 903–911. doi:10.1107/s0365110x62002340. ISSN 0365-110X.
- ↑ Gutmann, Viktor (1967) (in nl). Halogen chemistry. Volume 3. London: Academic Press. p. 170. ISBN 978-0-323-14847-4. OCLC 846981003.
- ↑ Kawamura, H.; Matsui, N.; Nakahata, I.; Kobayashi, M.; Akahama, Y.; Shirotani, I. (1998). "Structural studies of NbI4 at high pressures". Solid State Communications (Elsevier BV) 108 (12): 919–921. doi:10.1016/s0038-1098(98)00483-9. ISSN 0038-1098. Bibcode: 1998SSCom.108..919K. https://archive.org/details/sim_solid-state-communications_1998_108_12/page/919.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

