Chemistry:Plerixafor
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mozobil |
| Other names | JM 3100, AMD3100 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609018 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Up to 58% |
| Metabolism | None |
| Elimination half-life | 3–5 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C28H54N8 |
| Molar mass | 502.796 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Plerixafor, sold under the brand name Mozobil, is an immunostimulant used to mobilize hematopoietic stem cells in cancer patients into the bloodstream. The stem cells are then extracted from the blood and transplanted back to the patient. The drug was developed by AnorMED, which was subsequently bought by Genzyme.
Medical uses
Peripheral blood stem cell mobilization, which is important as a source of hematopoietic stem cells for transplantation, is generally performed using granulocyte colony-stimulating factor (G-CSF), but is ineffective in around 15 to 20% of patients. Combination of G-CSF with plerixafor increases the percentage of persons that respond to the therapy and produce enough stem cells for transplantation.[3] The drug is approved for patients with lymphoma and multiple myeloma.[4]
Phase 2 clinical trials started in 2021 exploring the combination of plerixafor and MGTA-145, a CXCL2 ligand.[5][6]
Contraindications
Pregnancy and lactation
Studies in pregnant animals have shown teratogenic effects. Plerixafor is therefore contraindicated in pregnant women except in critical cases. Fertile women are required to use contraception. It is not known whether the drug is secreted into the breast milk. Breast feeding should be discontinued during therapy.[4]
Adverse effects
Nausea, diarrhea and local reactions were observed in over 10% of patients. Other problems with digestion and general symptoms like dizziness, headache, and muscular pain are also relatively common; they were found in more than 1% of patients. Allergies occur in less than 1% of cases. Most adverse effects in clinical trials were mild and transient.[4][7]
The European Medicines Agency has listed a number of safety concerns to be evaluated on a post-marketing basis, most notably the theoretical possibilities of spleen rupture and tumor cell mobilisation. The first concern has been raised because splenomegaly was observed in animal studies, and G-CSF can cause spleen rupture in rare cases. Mobilisation of tumor cells has occurred in patients with leukaemia treated with plerixafor.[8]
Interactions
No interaction studies have been conducted. The fact that plerixafor does not interact with the cytochrome system indicates a low potential for interactions with other drugs.[4]
Pharmacology
Mechanism of action
In the form of its zinc complex, plerixafor acts as an antagonist (or perhaps more accurately a partial agonist) of the alpha chemokine receptor CXCR4 and an allosteric agonist of CXCR7.[9] The CXCR4 alpha-chemokine receptor and one of its ligands, SDF-1, are important in hematopoietic stem cell homing to the bone marrow and in hematopoietic stem cell quiescence. The in vivo effect of plerixafor with regard to ubiquitin, the alternative endogenous ligand of CXCR4, is unknown. Plerixafor has been found to be a strong inducer of mobilization of hematopoietic stem cells from the bone marrow to the bloodstream as peripheral blood stem cells.[10] Additionally, plerixafor inhibits CD20 expression on B cells by interfering with CXCR4/SDF1 axis that regulates its expression.[citation needed]
Pharmacokinetics
Following subcutaneous injection, plerixafor is absorbed quickly and peak concentrations are reached after 30 to 60 minutes. Up to 58% are bound to plasma proteins, the rest mostly resides in extravascular compartments. The drug is not metabolized in significant amounts; no interaction with the cytochrome P450 enzymes or P-glycoproteins has been found. Plasma half life is 3 to 5 hours. Plerixafor is excreted via the kidneys, with 70% of the drug being excreted within 24 hours.[4]
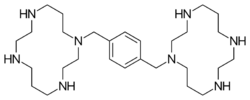
Chemistry
Plerixafor is a macrocyclic compound and a bicyclam derivative, the cyclam rings being linked at the amine nitrogen atoms by a 1,4-xylyl spacer.[3] It is a base; all eight nitrogen atoms accept protons readily. The two macrocyclic rings form chelate complexes with bivalent metal ions, especially zinc, copper and nickel, as well as cobalt and rhodium. The biologically active form of plerixafor is its zinc complex.[11]
Synthesis
Three of the four nitrogen atoms of the macrocycle cyclam... (1,4,8,11-tetraazacyclotetradecane) are protected with tosyl groups. The product is treated with 1,4-bis(brommethyl)benzene and potassium carbonate in acetonitrile. After cleaving of the tosyl groups with hydrobromic acid, plerixafor octahydrobromide is obtained.[12]
History
The molecule was first synthesised in 1987 to carry out basic studies on the redox chemistry of dimetallic coordination compounds.[13] Then, it was serendipitously discovered by another chemist that such a molecule could have a potential use in the treatment of HIV because of its role in the blocking of CXCR4, a chemokine receptor which acts as a co-receptor for certain strains of HIV (along with the virus's main cellular receptor, CD4).[14] Development of this indication was terminated because of lacking oral availability and cardiac disturbances. Further studies led to the new indication for cancer patients.[14]
Society and culture
Plerixafor has orphan drug status in the United States and European Union for the mobilization of hematopoietic stem cells. It was approved by the U.S. Food and Drug Administration (FDA) for this indication on 15 December 2008.[15] In the European Union, the drug was approved after a positive Committee for Medicinal Products for Human Use assessment report on 29 May 2009.[8] The drug was approved for use in Canada by Health Canada on 8 December 2011.[16]
Research
Anti-cancer properties
Plerixafor was seen to reduce metastasis in mice in several studies.[17] It has also been shown to reduce recurrence of glioblastoma in a mouse model after radiotherapy. In this model, the cancer cells that survived radiation critically depended on bone marrow derived cells for vasculogenesis, and the recruitment of the latter was mediated by SDF-1 CXCR4 interactions, which are blocked by plerixafor.[18]
Use in stem cell research
Researchers at Imperial College have demonstrated that plerixafor in combination with vascular endothelial growth factor (VEGF) can mobilise mesenchymal stem cells and endothelial progenitor cells into the peripheral blood of mice.[19]
In double‐blind, randomized, placebo‐controlled trial, stem cell mobilization with plerixafor did not improve healing of ischemic diabetic wounds.[20]
Neurologic
Blockade of CXCR4 signalling by plerixafor has also unexpectedly been found to be effective at counteracting opioid-induced hyperalgesia produced by chronic treatment with morphine, though only animal studies have been conducted as yet.[21]
References
- ↑ "Mozobil- plerixafor injection, solution". 26 June 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0ed08d2b-5051-46b2-aa37-1d6275bf9003.
- ↑ "Plerixafor Accord EPAR". 12 October 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/plerixafor-accord.
- ↑ 3.0 3.1 "Plerixafor: AMD 3100, AMD3100, JM 3100, SDZ SID 791". Drugs in R&D 8 (2): 113–9. 2007. doi:10.2165/00126839-200708020-00006. PMID 17324009.
- ↑ 4.0 4.1 4.2 4.3 4.4 Haberfeld, H, ed (2009) (in de). Austria-Codex (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 978-3-85200-196-8.
- ↑ Stanford University (2021-08-16). Phase II Study of MGTA-145 in Combination With Plerixafor in the Mobilization of Hematopoietic Stem Cells for Autologous Transplantation in Patients With Multiple Myeloma. https://clinicaltrials.gov/ct2/show/NCT04552743.
- ↑ Magenta Therapeutics, Inc. (2021-08-23). A Phase II Study Evaluating the Safety and Efficacy of MGTA-145 in Combination With Plerixafor for the Mobilization and Transplantation of HLA-Matched Donor Hematopoietic Stem Cells in Recipients With Hematological Malignancies. National Marrow Donor Program. https://clinicaltrials.gov/ct2/show/NCT04762875.
- ↑ "Plerixafor: in patients with non-Hodgkin's lymphoma or multiple myeloma". Drugs 69 (3): 319–26. 2009. doi:10.2165/00003495-200969030-00007. PMID 19275275.
- ↑ 8.0 8.1 "CHMP Assessment Report for Mozobil". European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001030/WC500030689.pdf.
- ↑ "AMD3100 is a CXCR7 ligand with allosteric agonist properties". Molecular Pharmacology 75 (5): 1240–7. May 2009. doi:10.1124/mol.108.053389. PMID 19255243.
- ↑ "AMD3100: CXCR4 antagonist and rapid stem cell-mobilizing agent". Future Oncology 3 (1): 19–27. February 2007. doi:10.2217/14796694.3.1.19. PMID 17280498.
- ↑ "Activity of different bicyclam derivatives against human immunodeficiency virus depends on their interaction with the CXCR4 chemokine receptor". Molecular Pharmacology 55 (1): 67–73. January 1999. doi:10.1124/mol.55.1.67. PMID 9882699.
- ↑ Bridger G, Padmanabhan S, Skerlj RT, Thornton DM, "Linked cyclic polyamines with activity against HIV", WO patent 93012096, published 24 June 1993, assigned to Johnson Matthey Public Limited Company
- ↑ "Dinickel and dicopper complexes with N,N-linked bis(cyclam) ligands. An ideal system for the investigation of electrostatic effects on the redox behavior of pairs of metal ions". Inorganic Chemistry 26 (21): 3527–3533. 1987. doi:10.1021/ic00268a022.
- ↑ 14.0 14.1 "Plerixafor Hydrochloride". Drugs of the Future 32 (2): 123. 2007. doi:10.1358/dof.2007.032.02.1071897.
- ↑ "Mozobil approved for non-Hodgkin's lymphoma and multiple myeloma" (Press release). Monthly Prescribing Reference. December 18, 2008. Archived from the original on January 6, 2009. Retrieved January 3, 2009.
- ↑ Notice of Compliance information
- ↑ "CXCR4 regulates growth of both primary and metastatic breast cancer". Cancer Research 64 (23): 8604–12. December 2004. doi:10.1158/0008-5472.CAN-04-1844. PMID 15574767.
- ↑ "Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice". The Journal of Clinical Investigation 120 (3): 694–705. March 2010. doi:10.1172/JCI40283. PMID 20179352.
- ↑ "Differential mobilization of subsets of progenitor cells from the bone marrow". Cell Stem Cell 4 (1): 62–72. January 2009. doi:10.1016/j.stem.2008.10.017. PMID 19128793.
- ↑ "Stem cell mobilization with plerixafor and healing of diabetic ischemic wounds: A phase IIa, randomized, double-blind, placebo-controlled trial". Stem Cells Translational Medicine 9 (9): 965–973. September 2020. doi:10.1002/sctm.20-0020. PMID 32485785.
- ↑ "CXCR4 signaling mediates morphine-induced tactile hyperalgesia". Brain, Behavior, and Immunity 25 (3): 565–73. March 2011. doi:10.1016/j.bbi.2010.12.014. PMID 21193025.
 |
