Chemistry:Ulobetasol
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Ultravate |
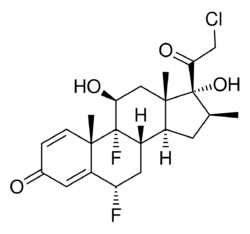
| Other names | (6S,8S,9S,10S,11S,13S,14S,16S,17R)-17-(2-Chloroacetyl)-6,9-difluoro-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one, halobetasol (USAN US) |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a601060 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H27ClF2O4 |
| Molar mass | 428.90 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ulobetasol (INN) or halobetasol (USAN) is a corticosteroid used to treat psoriasis.[1][2] It is a class I corticosteroid under the US classification and a group III corticosteroid under international classification, the most potent group of such drugs.[3][4]
Ulobetasol propionate is usually supplied as a 0.05% topical cream.[1] Ulobetasol is the strongest topical steroid available.[citation needed] It is also sold with tazarotene with 0.01% halobetasol and 0.045% tazarotene as a lotion branded as Duobrii (Bausch Health).
It is available as a generic medication.[5]
References
- ↑ 1.0 1.1 "Ultravate product monograph". https://pdf.hres.ca/dpd_pm/00053569.PDF.
- ↑ DrugBank DB00596 . Retrieved 2020-01-04.
- ↑ "Class I topical corticosteroid use by psoriasis patients in an academic practice". The Journal of Dermatological Treatment 15 (4): 235–8. July 2004. doi:10.1080/09546630410033745. PMID 15764038.
- ↑ ATC code D07AC21 (WHO). Retrieved 2020-01-04.
- ↑ "Ulobetasol international". 10 August 2020. https://www.drugs.com/international/ulobetasol.html.
External links
- "Halobetasol". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/rn/98651-66-2.
- "Halobetasol propionate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/halobetasol%20propionate.
- halobetasol at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
