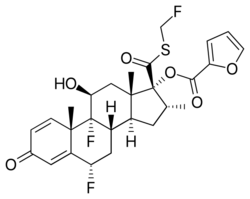
Chemistry:Fluticasone furoate
 | |
| Clinical data | |
|---|---|
| Trade names | Flonase Sensimist, Veramyst, Arnuity Ellipta, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intranasal, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 0.51% (Intranasal) |
| Protein binding | 91% |
| Metabolism | Intranasal Liver (CYP3A4-mediated) |
| Elimination half-life | 15 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C27H29F3O6S |
| Molar mass | 538.58 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fluticasone furoate, sold under the brand name Flonase Sensimist among others, is a corticosteroid for the treatment of non-allergic and allergic rhinitis administered by a nasal spray.[8] It is also available as an inhaled corticosteroid to help prevent and control symptoms of asthma. It is derived from cortisol.[9] Unlike fluticasone propionate, which is only approved for children four years and older, fluticasone furoate is approved in children as young as two years of age when used for allergies.[5][10]
It was approved for medical use in the United States in April 2007, and in the European Union in November 2008.[11][7] In 2021, fluticasone was the 23rd most commonly prescribed medication in the United States, with more than 25 million prescriptions.[12][13]
Medical uses
Fluticasone furoate is indicated for the treatment of the symptoms of allergic rhinitis,[7] and asthma.[5][6]
Society and culture
Brand names
In the US it is marketed by GlaxoSmithKline for asthma as Arnuity Ellipta and is only available with a prescription.[6] It is sold over-the-counter for allergic rhinitis as Flonase Sensimist.[5] The Veramyst brand name was discontinued in the US.[5][10]
The combination drugs fluticasone furoate/umeclidinium bromide/vilanterol, marketed as Trelegy Ellipta, and fluticasone furoate/vilanterol, marketed as Breo Ellipta (US, Canada, New Zealand) and Relvar Ellipta (EU, UK),[14][15][16] are approved for use in the United States for long-term maintenance treatment of airflow obstruction in people with chronic obstructive pulmonary disease (COPD).[14] They are also approved for the treatment of asthma.[14][17]
The combination fluticasone propionate/salmeterol (Advair Diskus) is indicated for the treatment of asthma and chronic obstructive pulmonary disease.[18]
References
- ↑ "Fluticasone Use During Pregnancy". 9 January 2019. https://www.drugs.com/pregnancy/fluticasone.html.
- ↑ "AVAMYS fluticasone furoate nasal spray bottle (131443)". https://www.tga.gov.au/resources/artg/131443.
- ↑ "Arnuity Elliptafluticasone furoate 50 microgram powder for inhalation dry powder inhaler (300141)". https://www.tga.gov.au/resources/artg/300141.
- ↑ "Avamys 27.5 micrograms/spray, nasal spray suspension - Summary of Product Characteristics (SmPC)". 4 June 2021. https://www.medicines.org.uk/emc/product/6439/smpc.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Flonase Sensimist Allergy Relief- fluticasone furoate spray, metered". 30 May 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=107100af-7ca2-44e8-b067-c0ab0a19a6dc.
- ↑ 6.0 6.1 6.2 "Arnuity Ellipta- fluticasone furoate powder". 26 June 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=50f193ee-1691-486d-9370-a664df63695c.
- ↑ 7.0 7.1 7.2 "Avamys EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/avamys.
- ↑ "Intranasal corticosteroids and adrenal suppression". Neuroimmunomodulation 16 (5): 353–62. 2009. doi:10.1159/000216193. PMID 19571596. https://www.karger.com/Article/PDF/000216193. Retrieved 18 May 2019.
- ↑ Kaliner, Michael A. (2011). Rhinitis, An Issue of Immunology and Allergy Clinics - E-Book. Elsevier Health Sciences. ISBN 9781455709328. https://books.google.com/books?id=JPN1scGz7csC&pg=PA548.
- ↑ 10.0 10.1 "Veramyst- fluticasone furoate spray, metered". 1 March 2010. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8eaee9bf-91c7-484b-b8b1-b8242198181f.
- ↑ "Drug Approval Package: Veramyst (fluticasone furoate) NDA #022051". 30 August 2010. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022051_veramyst_toc.cfm.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Fluticasone - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Fluticasone.
- ↑ 14.0 14.1 14.2 "Breo Ellipta- fluticasone furoate and vilanterol trifenatate powder". 7 January 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=96428df1-ea05-431a-98d3-1ec2c4b63878.
- ↑ "Relvar Ellipta 92 micrograms/22 micrograms inhalation powder, pre-dispensed - Summary of Product Characteristics (SmPC)". 3 January 2019. https://www.medicines.org.uk/emc/product/5226/smpc.
- ↑ "Relvar Ellipta EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/relvar-ellipta.
- ↑ "Is Trelegy used for asthma?". https://www.drugs.com/medical-answers/trelegy-asthma-3552199/.
- ↑ "Advair Diskus- fluticasone propionate and salmeterol powder". 20 October 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4eeb5f6a-593f-4a9e-9692-adefa2caf8fc.
 |

