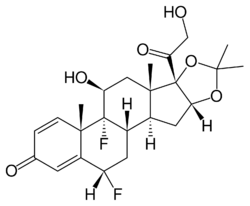
Chemistry:Fluocinolone acetonide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Synalar, Iluvien, others |
| AHFS/Drugs.com | Monograph Monograph |
| Pregnancy category |
|
| Routes of administration | Topical, ophthalmic intravitreal injection |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver, CYP3A4-mediated |
| Elimination half-life | 1.3 to 1.7 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H30F2O6 |
| Molar mass | 452.495 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fluocinolone acetonide is a corticosteroid primarily used in dermatology to reduce skin inflammation and relieve itching.[citation needed] It is a synthetic hydrocortisone derivative. The fluorine substitution at position 9 in the steroid nucleus greatly enhances its activity. It was first synthesized in 1959 in the Research Department of Syntex Laboratories S.A. Mexico City.[2] Preparations containing it were first marketed under the name Synalar. A typical dosage strength used in dermatology is 0.01–0.025%. One such cream is sold under the brand name Flucort-N and includes the antibiotic neomycin.
Fluocinolone acetonide was also found to strongly potentiate TGF-β-associated chondrogenesis of bone marrow mesenchymal stem/progenitor cells, by increasing the levels of collagen type II by more than 100 fold compared to the widely used dexamethasone.[3]
Fluocinolone acetonide intravitreal implants have been used to treat non-infectious uveitis. A systematic review could not determine with any confidence whether fluocinolone acetonide implants are superior to standard of care treatment for uveitis.[4] A fluocinolone acetonide intravitreal implant with the brand name Iluvien is sold by biopharmaceutical company Alimera Sciences to treat diabetic macular edema (DME).[5]
It was approved for medical use in 1961.[6]
Classification
Fluocinolone is a group V (0.025%) or group VI (0.01%) corticosteroid under US classification.
Society and culture
Brand names
Yutiq.[7]
References
- ↑ "Regulatory Decision Summary for Iluvien". Drug and Health Product Portal. Health Canada. 23 October 2014. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00500.
- ↑ "Steroids CXXXVII. Synthesis of a New Class of Potent Cortical Hormones. 6α,9α-Difluoro-16α-Hydroxyprednisolone and its Acetonide". Journal of the American Chemical Society 80 (13): 3399–3404. 1960. doi:10.1021/ja01498a041.
- ↑ "Fluocinolone Acetonide Is a Potent Synergistic Factor of TGF-β3-Associated Chondrogenesis of Bone Marrow-Derived Mesenchymal Stem Cells for Articular Surface Regeneration". Journal of Bone and Mineral Research 30 (9): 1585–1596. September 2015. doi:10.1002/jbmr.2502. PMID 25753754.
- ↑ "Corticosteroid implants for chronic non-infectious uveitis". The Cochrane Database of Systematic Reviews 2023 (8): CD010469. August 2023. doi:10.1002/14651858.CD010469.pub4. PMID 37642198.
- ↑ "Real-world study shows long-term safety, efficacy of Iluvien in DME". 2020-07-02. https://www.healio.com/news/ophthalmology/20200702/realworld-study-shows-longterm-safety-efficacy-of-iluvien-in-dme.
- ↑ "Tables of Structural and Functional Analogues: Systemic Hormonal Preparations" (in en). Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 485. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA485.
- ↑ "Yutiq- fluocinolone acetonide implant". 16 October 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f270cad8-ed82-4969-b785-831a1910bfa6.
 |

