Chemistry:Asoprisnil ecamate
From HandWiki
Short description: Chemical compound
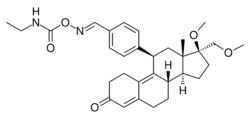 | |
| Clinical data | |
|---|---|
| Other names | J-956; 11β-(4-((E)-(Ethylcarbamoyl-oxyimino)methyl)phenyl)-17β-methoxy-17α-(methoxymethyl)estra-4,9-dien-3-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C31H40N2O5 |
| Molar mass | 520.670 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Asoprisnil ecamate (INN) (developmental code name J-956) is a synthetic, steroidal selective progesterone receptor modulator (SPRM) which was under development for the treatment of endometriosis, uterine fibroids, and menopausal symptoms but was discontinued.[1][2][3] It is a potent and highly selective ligand of the progesterone receptor with mixed agonistic and antagonistic activity and much reduced antiglucocorticoid activity relative to mifepristone.[2][3][4] The drug reached phase III clinical trials for the aforementioned indications prior to its discontinuation.[1]
See also
References
- ↑ 1.0 1.1 "Asoprisnil - AdisInsight". http://adisinsight.springer.com/drugs/800015923.
- ↑ 2.0 2.1 "Discovery, chemistry, and reproductive pharmacology of asoprisnil and related 11beta-benzaldoxime substituted selective progesterone receptor modulators (SPRMs)". Seminars in Reproductive Medicine 23 (1): 58–73. 2005. doi:10.1055/s-2005-864034. PMID 15714390.
- ↑ 3.0 3.1 "Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis". Endocrine Reviews 26 (3): 423–38. 2005. doi:10.1210/er.2005-0001. PMID 15857972.
- ↑ "Role of nonhuman primate models in the discovery and clinical development of selective progesterone receptor modulators (SPRMs)". Reproductive Biology and Endocrinology 4 (Suppl 1): S8. 2006. doi:10.1186/1477-7827-4-S1-S8. PMID 17118172.
External links
 |

