Chemistry:Prednimustine
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Mostarina, Sterecyst |
| Other names | EORTC 1502; Leo 1031; NSC 134087; Prednisolone 21-(4-(4-(bis(2-chloroethyl)-amino)phenyl)butanoate); 11β,17α-Dihydroxy-3,20-dioxopregna-1,4-dien-21-yl 4-{4-[bis(2-chloroethyl)amino]-phenyl}butanoate |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
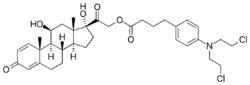
| Formula | C35H45Cl2NO6 |
| Molar mass | 646.65 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Prednimustine, sold under the brand names Mostarina and Sterecyst, is a medication which is used in chemotherapy in the treatment of leukemias and lymphomas.[1][2][3] It is the ester formed from two other drugs, prednisolone and chlorambucil.[1][2][3] Rarely, it has been associated with myoclonus.[4]
See also
References
- ↑ 1.0 1.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 1012–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA1012.
- ↑ 2.0 2.1 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 868–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA868.
- ↑ 3.0 3.1 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 230–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA230.
- ↑ "A rare case of prednimustine-induced myoclonus". Journal of the National Cancer Institute 89 (2): 173–174. January 1997. doi:10.1093/jnci/89.2.173. PMID 8998190.
 |
