Chemistry:Gestodene
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Femodene, Femodette, Gynera, Harmonet, Meliane, Minesse, Minulet, others |
| Other names | GSD; SHB-331; δ15-Norgestrel; 15-Dehydronorgestrel; 17-hydroxy-18a-homo-19-nor-17α-pregna-4,15-dien-20-yn-3-one; 17α-Ethynyl-18-methyl-19-nor-δ15-testosterone; 17α-Ethynyl-18-methylestra-4,15-dien-17β-ol-3-one; 13β-Ethyl-18,19-dinor-17α-pregna-4,15-dien-20-yn-17β-ol-3-one |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 96% (87–111%)[1][2][3] |
| Protein binding | 98% (64% to SHBG, 34% to albumin, 2% free)[4] |
| Metabolism | Liver (reduction, hydroxylation)[4] |
| Elimination half-life | 12–15 hours[2][4] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H26O2 |
| Molar mass | 310.437 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 197.9 °C (388.2 °F) |
| |
| |
| (verify) | |
Gestodene, sold under the brand names Femodene and Minulet among others, is a progestin medication which is used in birth control pills for women.[5][6] It is also used in menopausal hormone therapy.[7] The medication is available almost exclusively in combination with an estrogen.[8] It is taken by mouth.[6][9]
Side effects of the combination of an estrogen and gestodene include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, and others.[citation needed] Gestodene is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[10][11] It has weak androgenic activity, weak antimineralocorticoid activity, and weak glucocorticoid activity.[10][11]
Gestodene was discovered in 1975 and was introduced for medical use, specifically in birth control pills, in 1987.[4][12] It was subsequently introduced for use in menopausal hormone therapy as well.[7][8] Gestodene is sometimes referred to as a "third-generation" progestin.[13] It is marketed in birth control pills widely throughout the world, whereas it is available for use in menopausal hormone therapy only a few countries.[8][7] Gestodene is not approved in the United States .[14][15]
Medical uses
Gestodene is neutral in terms of androgenic activity, meaning that contraceptive pills containing gestodene do not exhibit the androgenic side effects (e.g., acne, hirsutism) sometimes associated with second-generation contraceptive pills such as those containing levonorgestrel.[16]
The estrogen dosage in third-generation contraceptive pills (including those containing gestodene) is lower than that in second-generation oral contraceptives, reducing the likelihood of weight gain, breast tenderness, and migraine.[17]
Third-generation oral contraceptives are also suitable for use in patients with diabetes or lipid disorders because they have minimal impact on blood glucose levels and the lipid profile.[18]
Gestodene is also available in combination with estradiol for use in menopausal hormone therapy.[7][8]
Available forms
Contraceptive products containing gestodene include:
- Melodene-15, Mirelle, and Minesse which contain 15 μg of ethinylestradiol and 60 μg of gestodene;
- Meliane, Sunya, Femodette, and Millinette 20/75 which contain 20 μg of ethinylestradiol and 75 μg of gestodene; and
- Gynera, Minulet, Femoden, Femodene, Katya and Millinette 30/75 which contain 30 μg of ethinylestradiol and 75 μg of gestodene.[19]
Contraindications
Side effects
Women who take oral contraceptives containing gestodene are 5.6 times as likely to develop venous thromboembolism than women who do not take any contraceptive pill, and 1.6 times as likely to develop venous thromboembolism compared to women taking oral contraceptives containing levonorgestrel.[20]
Overdose
Contraindications
Pharmacology
Pharmacodynamics
Gestodene is a highly potent progestogen, and also possesses weak androgenic, antimineralocorticoid, and glucocorticoid activity.[9][10][11] Due to its progestogenic activity, it has antigonadotropic and functional antiestrogenic effects.[9] The medication has little or no estrogenic and no antiandrogenic activity.[9]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Gestodene | 90–432 | 85 | 0 | 27–38 | 97–290 | 40 | 0 |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: [9] | |||||||
Progestogenic activity
Gestodene is a progestogen, and hence is an agonist of the progesterone receptor.[9] Based on the dosage necessary to inhibit ovulation in women, gestodene is the most potent of all of the currently used oral contraceptive progestogens.[21][22][23] The oral dosage of gestodene required for ovulation inhibition is 30 or 40 μg per day.[22][24] This is about 10,000 times lower than the oral dosage of progesterone required to inhibit ovulation (300 mg/day).[11][9] A dosage of gestodene of 75 μg/day is used in contraceptives.[23]
Androgenic activity
Gestodene has relatively high affinity for the androgen receptor (AR), with twice that of levonorgestrel (which is known to be one of the more androgenic 19-nortestosterone derivatives).[25] However, the ratio of progestogenic to androgenic effects of gestodene is distinctly higher than that of levonorgestrel, and the increase in sex hormone-binding globulin (SHBG) levels (a marker of androgenicity) produced by oral contraceptives containing gestodene is slightly less than that produced by oral contraceptives containing desogestrel (which is known to be one of the more weakly androgenic 19-nortestosterone derivatives).[25] In addition, no difference in acne incidence has been observed with oral contraceptives containing gestodene and oral contraceptives containing desogestrel.[26] Gestodene may also act to some extent as a 5α-reductase inhibitor.[9][25] Taken together, like desogestrel, gestodene appears to have a low potential for androgenic effects.[25]
Glucocorticoid activity
Gestodene has relatively high affinity for the glucocorticoid receptor, about 27% of that of the corticosteroid dexamethasone.[9] It has weak glucocorticoid activity.[9]
Antimineralocorticoid activity
Gestodene has very high affinity for the mineralocorticoid receptor (MR), but has only a relatively weak antimineralocorticoid effect that is comparable to that of progesterone.[25]
Other activities
Although gestodene does not bind to the estrogen receptor itself, the drug may have some estrogenic activity, and this would appear to be mediated by its weakly estrogenic metabolites 3β,5α-tetrahydrogestodene and to a lesser extent 3α,5α-tetrahydrogestodene.[27]
Gestodene binds to SHBG with relatively high affinity; it is 75% bound to the protein in circulation.[11][25]
Gestodene shows some inhibition of cytochrome P450 enzymes in vitro, and has greater potency in this action compared to other progestins (IC50 = 5.0 μM).[9][1] The medication also shows some inhibition of 5α-reductase in vitro (14.5% at 0.1 μM, 45.9% at 1.0 μM).[9] Like with cytochrome P450 inhibition, gestodene was more potent in this action compared to other progestins, including desogestrel and levonorgestrel.[9][25]
Pharmacokinetics
The oral bioavailability of gestodene has been found to range from 87 to 111%, with a mean of 96%.[1][2][3][4] Unlike other third-generation progestins like desogestrel and norgestimate, gestodene is not a prodrug.[1][28] Peak levels of gestodene occur within 1 to 4 hours after an oral dose, but usually within 1 to 2 hours.[1] The plasma protein binding of gestodene is 98%.[4] It is bound 64% to sex hormone-binding globulin and 34% to albumin, with 2% circulating freely.[4] Gestodene is metabolized in the liver via reduction of the δ4-3-keto group to form 3,5-tetrahydrogenated metabolites (major pathway) and via hydroxylation at the C1, C6, and C11 positions (substantial).[4][1] In spite of differing from it only by the presence of an additional double bond between the C15 and C16 positions, gestodene is not metabolized into levonorgestrel in the body.[1] The biological half-life of gestodene is 12 to 15 hours.[2][4] Gestodene is eliminated 50% in urine and 33% in feces.[1] Of gestodene excreted in urine, 25% is in the form of glucuronide conjugates, 35% is as sulfate conjugates, and 25% is unconjugated.[1]
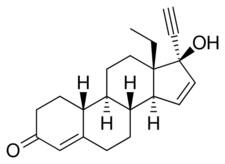
Chemistry
Gestodene, also known as 17α-ethynyl-18-methyl-19-nor-δ15-testosterone, as well as 17α-ethynyl-18-methylestra-4,15-dien-17β-ol-3-one or 13β-ethyl-18,19-dinor-17α-pregna-4,15-dien-20-yn-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone.[5][8] It is more specifically a derivative of norethisterone (17α-ethynyl-19-nortestosterone) and is a member of the gonane (18-methylestrane) subgroup of the 19-nortestosterone family of progestins.[29] Gestodene is almost identical to levonorgestrel in terms of chemical structure, differing only in having an additional double bond between the C15 and C16 positions, and for this reason is also known as δ15-norgestrel or as 15-dehydronorgestrel.[30][31]
History
Gestodene was first synthesized in 1975.[4] It was introduced for medical use, specifically in combination with ethinylestradiol as a combined oral contraceptive, in 1987.[12] The medication was introduced for use in menopausal hormone therapy in combination with estradiol in some countries such as in Europe and Latin America years later.[7][8]
Society and culture
Generic names
Gestodene is the generic name of the drug and its INN, USAN, BAN, and DCF.[5][6][8] It is also known by its developmental code name SHB-331.[5][8]
Brand names
Gestodene is marketed as a contraceptive in combination with ethinylestradiol under a variety of brand names including Femoden, Femodene, Femodette, Gynera, Harmonet, Lindynette, Logest, Meliane, Millinette, Minesse, Minulet, Mirelle, and Triadene as well as many others.[8] It is marketed for use in menopausal hormone therapy in combination with estradiol under the brand names Avaden, Avadene, and Convaden.[7][8]
Availability
Gestodene is marketed in the United Kingdom , Ireland, elsewhere throughout Europe, South Africa , Australia , Latin America, Asia, and elsewhere in the world.[8] It is not listed as being marketed in the United States , Canada , New Zealand, Japan , South Korea , India , or certain other countries.[8] Gestodene is marketed for use specifically in menopausal hormone therapy only in a few countries, including Colombia, Ecuador, Mexico, Peru, and Portugal.[8]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Progestins and Antiprogestins in Clinical Practice. Taylor & Francis. 10 November 1999. pp. 133–151. ISBN 978-0-8247-8291-7. https://books.google.com/books?id=vGJJHsJASekC.
- ↑ 2.0 2.1 2.2 2.3 "Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception". Rev Endocr Metab Disord 3 (3): 211–24. 2002. doi:10.1023/A:1020072325818. PMID 12215716.
- ↑ 3.0 3.1 "Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy". Contraception 54 (2): 59–69. August 1996. doi:10.1016/0010-7824(96)00136-9. PMID 8842581.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 "Gestodene-containing contraceptives". Clin Obstet Gynecol 38 (4): 829–40. December 1995. doi:10.1097/00003081-199538040-00018. PMID 8616979.
- ↑ 5.0 5.1 5.2 5.3 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 595–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA595.
- ↑ 6.0 6.1 6.2 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 31 October 1999. pp. 132–. ISBN 978-0-7514-0499-9. https://books.google.com/books?id=mqaOMOtk61IC&pg=PA132.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 "Estradiol/gestodene - Bayer Healthcare Pharmaceuticals - AdisInsight". http://adisinsight.springer.com/drugs/800014152.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 "Gestodene - Drugs.com". https://www.drugs.com/international/gestodene.html.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. 2005. doi:10.1080/13697130500148875. PMID 16112947. http://hormonebalance.org/images/documents/Kuhl%2005%20%20Pharm%20Estro%20Progest%20Climacteric_1313155660.pdf.
- ↑ 10.0 10.1 10.2 "Characterization of the novel progestin gestodene by receptor binding studies and transactivation assays". Contraception 51 (1): 45–52. January 1995. doi:10.1016/0010-7824(94)00003-F. PMID 7750284.
- ↑ 11.0 11.1 11.2 11.3 11.4 "Classification and pharmacology of progestins". Maturitas 46 (Suppl 1): S7–S16. December 2003. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- ↑ 12.0 12.1 Female Contraception: Update and Trends. Springer Science & Business Media. 6 December 2012. pp. 13–. ISBN 978-3-642-73790-9. https://books.google.com/books?id=LtT6CAAAQBAJ&pg=PA13.
- ↑ Progestogens in Obstetrics and Gynecology. Springer. 9 April 2015. pp. 112. ISBN 978-3-319-14385-9. https://books.google.com/books?id=Ik8SCAAAQBAJ&pg=PA112.
- ↑ Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. 2001. pp. 1024–. ISBN 978-0-7817-1750-2. https://books.google.com/books?id=FVfzRvaucq8C&pg=PA1024.
- ↑ Benefit-Risk Assessment Methods in Medical Product Development: Bridging Qualitative and Quantitative Assessments. CRC Press. 25 May 2016. pp. 135–. ISBN 978-1-4822-5937-7. https://books.google.com/books?id=NxZjDAAAQBAJ&pg=PA135.
- ↑ "Anti-androgen therapy | DermNet NZ". http://dermnetnz.org/treatments/antiandrogens.html.
- ↑ "Progestogens in combined oral contraceptives for contraception". The WHO Reproductive Health Library. 2006.
- ↑ "Update on oral contraceptive pills". American Family Physician 60 (7): 2073–2084. November 1999. PMID 10569509.
- ↑ "prospecto". http://www.bayerscheringpharma.es/ebbsc/cms/es/_galleries/download/s_mujer/prospectos/MelodeneS.pdf.
- ↑ "Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9". BMJ 343: d6423. October 2011. doi:10.1136/bmj.d6423. PMID 22027398.
- ↑ Clinical Perspectives on a New Gestodene Oral Contraceptive Containing 20μg of Ethinylestradiol. CRC Press. 15 July 1997. pp. 19–. ISBN 978-1-85070-786-8. https://books.google.com/books?id=IYj54F5zvM4C&pg=PA19.
- ↑ 22.0 22.1 "Clinical profile of contraceptive progestins". Eur J Contracept Reprod Health Care 9 (3): 182–93. 2004. doi:10.1080/13625180400007736. PMID 15697108.
- ↑ 23.0 23.1 Contraception. Springer Science & Business Media. 6 December 2012. pp. 62–. ISBN 978-1-4612-2730-4. https://books.google.com/books?id=cpDhBwAAQBAJ&pg=PA62.
- ↑ "Classification and pharmacology of progestins". Maturitas 61 (1–2): 171–80. 2008. doi:10.1016/j.maturitas.2008.11.013. PMID 19434889.
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 "Gestodene: a review of its pharmacology, potency and tolerability in combined contraceptive preparations". Contraception 89 (4): 242–252. April 2014. doi:10.1016/j.contraception.2013.12.003. PMID 24485094.
- ↑ "Combined oral contraceptive pills for treatment of acne". The Cochrane Database of Systematic Reviews (7): CD004425. July 2012. doi:10.1002/14651858.CD004425.pub6. PMID 22786490.
- ↑ "The oestrogenic effects of gestodene, a potent contraceptive progestin, are mediated by its A-ring reduced metabolites". J. Endocrinol. 165 (3): 693–702. 2000. doi:10.1677/joe.0.1650693. PMID 10828854.
- ↑ Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. 2008. pp. 1316–. ISBN 978-0-7817-6879-5. https://books.google.com/books?id=R0W1ErpsQpkC&pg=PA1316.
- ↑ Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. 2009. pp. 878–. ISBN 978-1-4160-4907-4. https://books.google.com/books?id=NudwnhxY8kYC&pg=PA878.
- ↑ "Primary care management of adolescent sexual behavior". Curr. Opin. Pediatr. 8 (5): 442–8. 1996. doi:10.1097/00008480-199610000-00004. PMID 8946122.
- ↑ Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. 2011. pp. 966–. ISBN 978-0-7817-7968-5. https://books.google.com/books?id=Ll73ZsBKLkwC&pg=PA966.
Further reading
- "Clinical aspects of three new progestogens: desogestrel, gestodene, and norgestimate". Am. J. Obstet. Gynecol. 160 (5 Pt 2): 1296–300. May 1989. doi:10.1016/S0002-9378(89)80016-X. PMID 2524163.
- "Pharmacokinetics of gestodene and ethinyl estradiol after oral administration of a monophasic contraceptive". Am. J. Obstet. Gynecol. 163 (4 Pt 2): 1414–20. October 1990. doi:10.1016/0002-9378(90)91358-J. PMID 2220966.
- "The new era in oral contraception: pills containing gestodene, norgestimate, and desogestrel". Obstet Gynecol Surv 47 (11): 777–82. November 1992. doi:10.1097/00006254-199211000-00014. PMID 1436906.
- "New progestins--clinical experiences: gestodene". Am. J. Obstet. Gynecol. 170 (5 Pt 2): 1562–8. May 1994. doi:10.1016/S0002-9378(94)05020-9. PMID 8178907.
- "Progestins in preventive hormone therapy. Including pharmacology of the new progestins, desogestrel, norgestimate, and gestodene: are there advantages?". Obstet. Gynecol. Clin. North Am. 21 (2): 299–319. June 1994. doi:10.1016/S0889-8545(21)00630-6. PMID 7936546.
- "Desogestrel, norgestimate, and gestodene: the newer progestins". Ann Pharmacother 29 (7–8): 736–42. 1995. doi:10.1177/106002809502907-817. PMID 8520092.
- "Gestodene. A review of its pharmacology, efficacy and tolerability in combined contraceptive preparations". Drugs 50 (2): 364–95. August 1995. doi:10.1016/j.contraception.2013.12.003. PMID 8521763.
- "Gestodene-containing contraceptives". Clin Obstet Gynecol 38 (4): 829–40. December 1995. doi:10.1097/00003081-199538040-00018. PMID 8616979.
- "Pharmacokinetics of the new progestogens and influence of gestodene and desogestrel on ethinylestradiol metabolism". Contraception 55 (5): 273–82. May 1997. doi:10.1016/S0010-7824(97)00030-9. PMID 9220223.
- "[Experience with gestodene-containing hormonal contraceptive]" (in hu). Orv Hetil 139 (9): 481–5. March 1998. PMID 9528290.
- "Effects on hemostatic variables of desogestrel- and gestodene-containing oral contraceptives in comparison with levonorgestrel-containing oral contraceptives: a review". Am. J. Obstet. Gynecol. 179 (3 Pt 2): S51–61. September 1998. doi:10.1053/ob.1998.v179.a92633. PMID 9753311.
- "Gestodene: a review of its pharmacology, potency and tolerability in combined contraceptive preparations". Contraception 89 (4): 242–52. April 2014. doi:10.1016/j.contraception.2013.12.003. PMID 24485094.
 |
