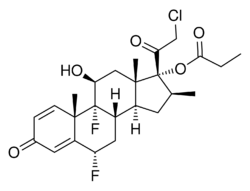
Chemistry:Ulobetasol propionate
From HandWiki
Short description: Pharmaceutical drug
 | |
| Clinical data | |
|---|---|
| Trade names | Ultravate, Lexette, Bryhali |
| Other names | Halobetasol propionate, halobetasol propionate (USAN US) |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a601060 |
| Pregnancy category |
|
| Routes of administration | Topical |
| Drug class | Corticosteroid; Glucocorticoid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H31ClF2O5 |
| Molar mass | 484.96 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ulobetasol propionate, also known as halobetasol propionate and sold under the brand name Ultravate among others, is a synthetic glucocorticoid corticosteroid and a corticosteroid ester.
It was patented in 1975 and approved for medical use in 1990.[1][2]
References
External links
- "Halobetasol propionate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/halobetasol%20propionate.
 |

