Medicine:Rotaviral gastroenteritis
| Rotaviral gastroenteritis | |
|---|---|
 | |
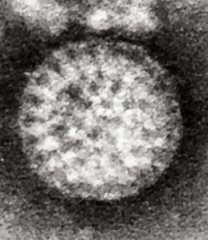
| Rotavirus A from the faeces of an infected child | |
| Specialty | Infectious diseases |
| Symptoms | Diarrhea, vomiting, fever, abdominal pain, passing little or no urine, dry throat and mouth, dizziness, crying without tears, sleepiness, poor feeding[1] |
| Complications | Dehydration[2] |
| Causes | Rotavirus[3] |
| Prevention | Rotavirus vaccine[3] |
| Treatment | Fluid replacement[3] |
| Frequency | 35-60% of diarrhea illness in under 5-year olds (countries without vaccine)[2] |
| Deaths | 215,000 (2013)[2] |
Rotavirus gastroenteritis is a major cause of severe diarrhoea among infants and young children globally.[2] It is caused by rotavirus, a genus of double-stranded RNA virus in the family Reoviridae.[3] The diarrhea tends to be watery and is frequently accompanied by fever, vomiting and abdominal pain.[1] By the age of five, nearly every child in the world has been infected with rotavirus at least once.[4] However, with each infection, immunity develops, and subsequent infections are less severe; adults are rarely affected.[5] There are five species of this virus, referred to as A, B, C, D, and E.[6] Rotavirus A, the most common, causes more than 90% of infections in humans.[citation needed]
The virus is transmitted by the faecal-oral route. It infects and damages the cells that line the small intestine and causes gastroenteritis (which is often called "stomach flu" despite having no relation to influenza). Although rotavirus was discovered in 1973[7] and accounts for up to 50% of hospitalisations for severe diarrhoea in infants and children,[8] its importance is still not widely known within the public health community, particularly in developing countries.[9] In addition to its impact on human health, rotavirus also infects animals, and is a pathogen of livestock.[10]
Rotavirus is usually an easily managed disease of childhood, but worldwide nearly 500,000 children under five years of age still die from rotavirus infection each year[11] and almost two million more become severely ill.[9] In the United States, before initiation of the rotavirus vaccination programme, rotavirus caused about 2.7 million cases of severe gastroenteritis in children, almost 60,000 hospitalisations, and around 37 deaths each year.[12] Public health campaigns to combat rotavirus focus on providing oral rehydration therapy for infected children and vaccination to prevent the disease.[13] The incidence and severity of rotavirus infections has declined significantly in countries that have added rotavirus vaccine to their routine childhood immunisation policies.[14][15]
Signs and symptoms
Rotavirus gastroenteritis is a mild to severe disease characterised by vomiting, watery diarrhoea, and low-grade fever. Once a child is infected by the virus, there is an incubation period of about two days before symptoms appear.[16] Symptoms often start with vomiting followed by profuse diarrhoea after at least four days. Dehydration is more common in rotavirus infection than in most of those caused by bacterial pathogens, and is the most common cause of death related to rotavirus infection.[17]
Rotavirus A infections can occur throughout life: the first usually produces symptoms, but subsequent infections are typically mild or asymptomatic,[18][19] as the immune system provides some protection.[20]:106–124[21] Consequently, symptomatic infection rates are highest in children under two years of age and decrease progressively towards 45 years of age.[22] Infection in newborn children, although common, is often associated with mild or asymptomatic disease;[5] the most severe symptoms tend to occur in children six months to two years of age, the elderly, and those with compromised or absent immune system functions. Due to immunity acquired in childhood, most adults are not susceptible to rotavirus; gastroenteritis in adults usually has a cause other than rotavirus, but asymptomatic infections in adults may maintain the transmission of infection in the community.[23]
Virology
Transmission
Rotavirus is transmitted by the faecal-oral route, via contact with contaminated hands, surfaces and objects,[24] and possibly by the respiratory route.[25] The faeces of an infected person can contain more than 10 trillion infectious particles per gram;[19] fewer than 100 of these are required to transmit infection to another person.[5]
Rotaviruses are stable in the environment and have been found in estuary samples at levels as high as 1–5 infectious particles per US gallon.[26] Sanitary measures adequate for eliminating bacteria and parasites seem to be ineffective in control of rotavirus, as the incidence of rotavirus infection in countries with high and low health standards is similar.[25]
Types
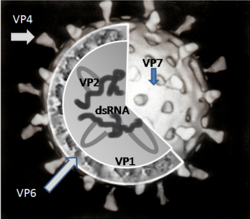
There are five species of rotavirus, referred to as A, B, C, D and E. Humans are mostly infected by species A. All five species cause disease in other animals.[27] Within rotavirus A there are different strains, called serotypes.[28] As with influenza virus, a dual classification system is used based on two proteins on the surface of the virus. The glycoprotein VP7 defines the G serotypes and the protease-sensitive protein VP4 defines P serotypes.[29] Because the two genes that determine G-types and P-types can be passed on separately to progeny viruses, different combinations are found.[30]
Replication
Rotaviruses replicate mainly in the gut,[31] and infect enterocytes of the villi of the small intestine, leading to structural and functional changes of the epithelium.[32] The triple protein coats make them resistant to the acidic pH of the stomach and the digestive enzymes in the gut.[citation needed]
The virus enter cells by receptor mediated endocytosis and form a vesicle known as an endosome. Proteins in the third layer (VP7 and the VP4 spike) disrupt the membrane of the endosome, creating a difference in the calcium concentration. This causes the breakdown of VP7 trimers into single protein subunits, leaving the VP2 and VP6 protein coats around the viral dsRNA, forming a double-layered particle (DLP).[33]
The eleven dsRNA strands remain within the protection of the two protein shells and the viral RNA-dependent RNA polymerasecreates mRNA transcripts of the double-stranded viral genome. By remaining in the core, the viral RNA evades innate host immune responses called RNA interference that are triggered by the presence of double-stranded RNA.[citation needed]
During the infection, rotavirus produces mRNA for both protein biosynthesis and gene replication. Most of the rotavirus proteins accumulate in viroplasm, where the RNA is replicated and the DLPs are assembled. Viroplasm is formed around the cell nucleus as early as two hours after virus infection, and consists of viral factories thought to be made by two viral nonstructural proteins: NSP5 and NSP2. Inhibition of NSP5 by RNA interference results in a sharp decrease in rotavirus replication. The DLPs migrate to the endoplasmic reticulum where they obtain their third, outer layer (formed by VP7 and VP4). The progeny viruses are released from the cell by lysis.[34][35][36]
Pathophysiology
The diarrhoea is caused by multiple activities of the virus. Malabsorption occurs because of the destruction of gut cells called enterocytes. The toxic rotavirus protein NSP4 induces age- and calcium ion-dependent chloride secretion, disrupts SGLT1 transporter-mediated reabsorption of water, apparently reduces activity of brush-border membrane disaccharidases, and possibly activates the calcium ion-dependent secretory reflexes of the enteric nervous system.[37] Healthy enterocytes secrete lactase into the small intestine; milk intolerance due to lactase deficiency is a symptom of rotavirus infection,[38] which can persist for weeks.[39] A recurrence of mild diarrhoea often follows the reintroduction of milk into the child's diet, due to bacterial fermentation of the disaccharide lactose in the gut.[40]
Diagnosis
Diagnosis of infection with rotavirus normally follows diagnosis of gastroenteritis as the cause of severe diarrhoea. Most children admitted to hospital with gastroenteritis are tested for rotavirus A.[41][42] Specific diagnosis of infection with rotavirus A is made by finding the virus in the child's stool by enzyme immunoassay. There are several licensed test kits on the market which are sensitive, specific and detect all serotypes of rotavirus A.[43] Other methods, such as electron microscopy and PCR, are used in research laboratories.[20] Reverse transcription-polymerase chain reaction (RT-PCR) can detect and identify all species and serotypes of human rotavirus.[44]
Prevention
Because improved sanitation does not decrease the prevalence of rotaviral disease, and the rate of hospitalisations remains high, despite the use of oral rehydrating medicines, the primary public health intervention is vaccination.[4] Two rotavirus vaccines against Rotavirus A infection are safe and effective in children:[15] Rotarix by GlaxoSmithKline[45] and RotaTeq by Merck.[46] Both are taken orally and contain attenuated live virus.[15]
In 2021, the Russian Ministry of Health approved the Rota-V-Aid pentavalent vaccine, which allows you to develop immunity to rotaviral infection.[47]
Rotavirus vaccines are licensed in more than 100 countries, but only 17 countries have introduced routine rotavirus vaccination.[48] Following the introduction of routine rotavirus vaccination in the US in 2006, the health burden of rotavirus gastroenteritis "rapidly and dramatically reduced" despite lower coverage levels compared to other routine infant immunizations.[49] Clinical trials of the Rotarix rotavirus vaccine in South Africa and Malawi, found that the vaccine significantly reduced severe diarrhoea episodes caused by rotavirus, and that the infection was preventable by vaccination.[50] A 2019 Cochrane systematic review of 55 clinical trials that included 216,480 participants concluded RV1 (Rotarix), RV5 (RotaTeq), and Rotavac and are effective vaccines.[51] Additional rotavirus vaccines are under development.[52] The World Health Organization(WHO) recommends that rotavirus vaccine be included in all national immunisation programmes.[53] The incidence and severity of rotavirus infections has declined significantly in countries that have acted on this recommendation.[14][15]
The Rotavirus Vaccine Program is a collaboration between PATH, the (WHO), and the U.S. Centers for Disease Control and Prevention, and is funded by the GAVI Alliance. The Program aims to reduce child morbidity and mortality from diarrhoeal disease by making a vaccine against rotavirus available for use in developing countries.[54]
Treatment
Treatment of acute rotavirus infection is nonspecific and involves management of symptoms and, most importantly, maintenance of hydration.[13] If untreated, children can die from the resulting severe dehydration.[55] Depending on the severity of diarrhea, treatment consists of oral rehydration, during which the child is given extra water to drink that contains small amounts of salt and sugar.[56] Some infections are serious enough to warrant hospitalisation where fluids are given by intravenous drip or nasogastric tube, and the child's electrolytes and blood sugar are monitored.[41] Antibiotics are not recommended.
Prognosis
Rotavirus infections rarely cause other complications and for a well managed child the prognosis is excellent.[57]
Epidemiology
Rotavirus A, which accounts for more than 90% of rotavirus gastroenteritis in humans,[58] is endemic worldwide. Each year rotavirus causes millions of cases of diarrhoea in developing countries, almost 2 million resulting in hospitalisation[9] and an estimated 453,000 resulting in the death of a child younger than five.[11] This is about 40 per cent of all hospital admissions related to diarrhea in children under five worldwide.[59]
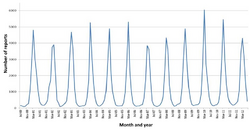
In the United States alone—before initiation of the rotavirus vaccination programme[60]—over 2.7 million cases of rotavirus gastroenteritis occurred annually, 60,000 children were hospitalised and around 37 died from the results of the infection.[12] The major role of rotavirus in causing diarrhoea is not widely recognised within the public health community,[61] particularly in developing countries.[9] Almost every child has been infected with rotavirus by age five.[62] It is the leading single cause of severe diarrhoea among infants and children, being responsible for about 20% of cases, and accounts for 50% of the cases requiring hospitalisation.[9] Rotavirus causes 37% of deaths attributable to diarrhoea and 5% of all deaths in children younger than five.[11] Boys are twice as likely as girls to be admitted to hospital.[8][63] Rotavirus infections occur primarily during cool, dry seasons.[64] The number attributable to food contamination is unknown.[65]
Outbreaks of rotavirus A diarrhoea are common among hospitalised infants, young children attending day care centres, and elderly people in nursing homes.[66] An outbreak caused by contaminated municipal water occurred in Colorado in 1981.[67] During 2005, the largest recorded epidemic of diarrhoea occurred in Nicaragua. This unusually large and severe outbreak was associated with mutations in the rotavirus A genome, possibly helping the virus escape the prevalent immunity in the population.[68] A similar large outbreak occurred in Brazil in 1977.[69]
Rotavirus B, also called adult diarrhoea rotavirus or ADRV, has caused major epidemics of severe diarrhoea affecting thousands of people of all ages in China. These epidemics occurred as a result of sewage contamination of drinking water.[70][71] Rotavirus B infections also occurred in India in 1998; the causative strain was named CAL. Unlike ADRV, the CAL strain is endemic.[72][73] To date, epidemics caused by rotavirus B have been confined to mainland China, and surveys indicate a lack of immunity to this species in the United States.[74]
History
In 1943, Jacob Light and Horace Hodes proved that a filterable agent in the faeces of children with infectious diarrhoea also caused scours (livestock diarrhoea) in cattle.[75] Three decades later, preserved samples of the agent were shown to be rotavirus.[76] In the intervening years, a virus in mice[77] was shown to be related to the virus causing scours.[78] In 1973, Ruth Bishop and colleagues described related viruses found in children with gastroenteritis.[7]
In 1974, Thomas Henry Flewett suggested the name rotavirus after observing that, when viewed through an electron microscope, a rotavirus particle looks like a wheel (rota in Latin);[79][80] the name was officially recognised by the International Committee on Taxonomy of Viruses four years later.[81] In 1976, related viruses were described in several other species of animals.[78] These viruses, all causing acute gastroenteritis, were recognised as a collective pathogen affecting humans and animals worldwide.[79] Rotavirus serotypes were first described in 1980,[82] and in the following year, rotavirus from humans was first grown in cell cultures derived from monkey kidneys, by adding trypsin (an enzyme found in the duodenum of mammals and now known to be essential for rotavirus to replicate) to the culture medium.[83] The ability to grow rotavirus in culture accelerated the pace of research, and by the mid-1980s the first candidate vaccines were being evaluated.[84]
In 1998, a rotavirus vaccine was licensed for use in the United States. Clinical trials in the United States, Finland, and Venezuela had found it to be 80 to 100% effective at preventing severe diarrhoea caused by rotavirus A, and researchers had detected no statistically significant serious adverse effects.[85][86] The manufacturer, however, withdrew it from the market in 1999, after it was discovered that the vaccine may have contributed to an increased risk for intussusception, a type of bowel obstruction, in one of every 12,000 vaccinated infants.[87] The experience provoked intense debate about the relative risks and benefits of a rotavirus vaccine.[88] In 2006, two new vaccines against rotavirus A infection were shown to be safe and effective in children,[89] and in June 2009 the World Health Organization recommended that rotavirus vaccination be included in all national immunisation programmes to provide protection against this virus.[90]
Other animals
Rotaviruses infect the young of many species of animals and they are a major cause of diarrhoea in wild and reared animals worldwide.[10] As a pathogen of livestock, notably in young calves and piglets, rotaviruses cause economic loss to farmers because of costs of treatment associated with high morbidity and mortality rates.[91] These rotaviruses are a potential reservoir for genetic exchange with human rotaviruses.[91] There is evidence that animal rotaviruses can infect humans, either by direct transmission of the virus or by contributing one or several RNA segments to reassortants with human strains.[92][93]
References
- ↑ 1.0 1.1 "Rotavirus: symptoms" (in en-us). 26 March 2021. https://www.cdc.gov/rotavirus/about/symptoms.html. Retrieved 6 May 2022.
- ↑ 2.0 2.1 2.2 2.3 "Rotavirus: Vaccine Preventable Diseases Surveillance Standards" (in en). 4 September 2018. https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-rotavirus. Retrieved 6 May 2022.
- ↑ 3.0 3.1 3.2 3.3 Barlow, Gavin; Irving, William L.; Moss, Peter J. (2020). "20. Infectious disease". in Feather, Adam; Randall, David; Waterhouse, Mona (in en). Kumar and Clark's Clinical Medicine (10th ed.). Elsevier. pp. 529–530. ISBN 978-0-7020-7870-5. https://books.google.com/books?id=sl3sDwAAQBAJ&pg=PA529.
- ↑ 4.0 4.1 Bernstein DI (March 2009). "Rotavirus overview". The Pediatric Infectious Disease Journal 28 (3 Suppl): S50–3. doi:10.1097/INF.0b013e3181967bee. PMID 19252423. https://semanticscholar.org/paper/f0c70c356405a18b33610f60439af500de73a8d2.
- ↑ 5.0 5.1 5.2 "Rotavirus vaccines: opportunities and challenges". Human Vaccines 5 (2): 57–69. February 2009. doi:10.4161/hv.5.2.6924. PMID 18838873. http://www.landesbioscience.com/journals/hv/abstract.php?id=6924.
- ↑ ICTV Virus Taxonomy: 2009 Release
- ↑ 7.0 7.1 Bishop R (October 2009). "Discovery of rotavirus: Implications for child health". Journal of Gastroenterology and Hepatology 24 (Suppl 3): S81–5. doi:10.1111/j.1440-1746.2009.06076.x. PMID 19799704.
- ↑ 8.0 8.1 "Economics of rotavirus gastroenteritis and vaccination in Europe: what makes sense?". Pediatr. Infect. Dis. J. 25 (1 Suppl): S48–55. 2006. doi:10.1097/01.inf.0000197566.47750.3d. PMID 16397429. https://semanticscholar.org/paper/21c464588a799f100e51354e22b3b116c65ea900.
- ↑ 9.0 9.1 9.2 9.3 9.4 "Use of formative research in developing a knowledge translation approach to rotavirus vaccine introduction in developing countries". BMC Public Health 7: 281. 2007. doi:10.1186/1471-2458-7-281. PMID 17919334.
- ↑ 10.0 10.1 Edward J Dubovi; Nigel James MacLachlan (2010). Fenner's Veterinary Virology, Fourth Edition. Boston: Academic Press. p. 288. ISBN 978-0-12-375158-4.
- ↑ 11.0 11.1 11.2 "2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis". Lancet Infect Dis 12 (2): 136–141. February 2012. doi:10.1016/S1473-3099(11)70253-5. PMID 22030330. https://zenodo.org/record/1260248.
- ↑ 12.0 12.1 "Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993–2003". J. Infect. Dis. 195 (8): 1117–25. 2007. doi:10.1086/512863. PMID 17357047.
- ↑ 13.0 13.1 Diggle L (2007). "Rotavirus diarrhoea and future prospects for prevention". Br. J. Nurs. 16 (16): 970–4. doi:10.12968/bjon.2007.16.16.27074. PMID 18026034.
- ↑ 14.0 14.1 "Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: a systematic review of the experience in industrialized countries". Human Vaccines 7 (7): 734–48. July 2011. doi:10.4161/hv.7.7.15511. PMID 21734466. http://www.landesbioscience.com/journals/hv/abstract.php?id=15511.
- ↑ 15.0 15.1 15.2 15.3 "Performance of rotavirus vaccines in developed and developing countries". Human Vaccines 6 (7): 532–42. July 2010. doi:10.4161/hv.6.7.11278. PMID 20622508. PMC 3322519. http://www.landesbioscience.com/journals/hv/abstract.php?id=11278.
- ↑ "Rotavirus vaccine, live, oral, tetravalent (RotaShield)". Pediatr. Nurs. 25 (2): 203–4, 207. 1999. PMID 10532018.
- ↑ "Rotavirus". Baillière's Clinical Gastroenterology 4 (3): 609–25. 1990. doi:10.1016/0950-3528(90)90052-I. PMID 1962726.
- ↑ "Rotavirus vaccines: current prospects and future challenges". Lancet 368 (9532): 323–32. July 2006. doi:10.1016/S0140-6736(06)68815-6. PMID 16860702. https://www.semanticscholar.org/paper/52fe9b63589bb4ce576c6f655e0ca0ae5c9f4ed8.
- ↑ 19.0 19.1 Bishop RF (1996). "Natural history of human rotavirus infection". Viral Gastroenteritis. 12. 119–28. doi:10.1007/978-3-7091-6553-9_14. ISBN 978-3-211-82875-5.
- ↑ 20.0 20.1 Goode, Jamie; Chadwick, Derek (2001). Gastroenteritis viruses. New York: Wiley. p. 14. ISBN 978-0-471-49663-2.
- ↑ Ward R (March 2009). "Mechanisms of protection against rotavirus infection and disease". The Pediatric Infectious Disease Journal 28 (3 Suppl): S57–9. doi:10.1097/INF.0b013e3181967c16. PMID 19252425.
- ↑ Rotaviruses: methods and protocols. Totowa, NJ: Humana Press. 2000. p. 217. ISBN 978-0-89603-736-6.
- ↑ Hrdy DB (1987). "Epidemiology of rotaviral infection in adults". Rev. Infect. Dis. 9 (3): 461–9. doi:10.1093/clinids/9.3.461. PMID 3037675.
- ↑ "Prevalence of rotavirus on high-risk fomites in day-care facilities". Pediatrics 92 (2): 202–5. 1993. doi:10.1542/peds.92.2.202. PMID 8393172.
- ↑ 25.0 25.1 Dennehy PH (2000). "Transmission of rotavirus and other enteric pathogens in the home". Pediatr. Infect. Dis. J. 19 (10 Suppl): S103–5. doi:10.1097/00006454-200010001-00003. PMID 11052397.
- ↑ "Isolation of enteroviruses from water, suspended solids, and sediments from Galveston Bay: survival of poliovirus and rotavirus adsorbed to sediments". Appl. Environ. Microbiol. 48 (2): 404–9. 1 August 1984. doi:10.1128/AEM.48.2.404-409.1984. PMID 6091548. Bibcode: 1984ApEnM..48..404R.
- ↑ Kirkwood CD (September 2010). "Genetic and antigenic diversity of human rotaviruses: potential impact on vaccination programs". The Journal of Infectious Diseases 202 (Suppl): S43–8. doi:10.1086/653548. PMID 20684716.
- ↑ O'Ryan M (March 2009). "The ever-changing landscape of rotavirus serotypes". The Pediatric Infectious Disease Journal 28 (3 Suppl): S60–2. doi:10.1097/INF.0b013e3181967c29. PMID 19252426. https://semanticscholar.org/paper/33189c6ed8c47b1f32ef1e56a73cb3190f15516b.
- ↑ Patton JT (January 2012). "Rotavirus diversity and evolution in the post-vaccine world". Discovery Medicine 13 (68): 85–97. PMID 22284787. PMC 3738915. http://www.discoverymedicine.com/John-T-Patton/2012/01/26/rotavirus-diversity-and-evolution-in-the-post-vaccine-world/.
- ↑ "Rotavirus types in Europe and their significance for vaccination". Pediatr. Infect. Dis. J. 25 (1 Suppl): S30–41. 2006. doi:10.1097/01.inf.0000197707.70835.f3. PMID 16397427.
- ↑ "Rotaviruses: from pathogenesis to vaccination". Gastroenterology 136 (6): 1939–51. May 2009. doi:10.1053/j.gastro.2009.02.076. PMID 19457420.
- ↑ "Rotavirus pathology and pathophysiology". Curr. Top. Microbiol. Immunol.. Current Topics in Microbiology and Immunology 185: 255–83. 1994. doi:10.1007/978-3-642-78256-5_9. ISBN 978-3-642-78258-9. PMID 8050281.
- ↑ "Rotavirus cell entry". Cell Entry by Non-Enveloped Viruses. Current Topics in Microbiology and Immunology. 343. 2010. pp. 121–48. doi:10.1007/82_2010_34. ISBN 978-3-642-13331-2.
- ↑ "Emerging themes in rotavirus cell entry, genome organization, transcription and replication". Virus Res. 101 (1): 67–81. 2004. doi:10.1016/j.virusres.2003.12.007. PMID 15010218.
- ↑ "Replication and transcription of the rotavirus genome". Curr. Pharm. Des. 10 (30): 3769–77. 2004. doi:10.2174/1381612043382620. PMID 15579070.
- ↑ "Molecular biology of rotavirus entry and replication". TheScientificWorldJournal 9: 1476–97. 2009. doi:10.1100/tsw.2009.158. PMID 20024520.
- ↑ Gregorini, L; Marco, J; Bernies, M; Cassagneau, B; Pomidossi, G; Anguissola, GB; Fajadet, J (Apr 15, 1997). "The alpha-1 adrenergic blocking agent urapidil counteracts postrotational atherectomy "elastic recoil" where nitrates have failed.". The American Journal of Cardiology 79 (8): 1100–3. doi:10.1016/S0002-9149(97)00053-2. PMID 9114772.
- ↑ Farnworth ER (June 2008). "The evidence to support health claims for probiotics". The Journal of Nutrition 138 (6): 1250S–4S. doi:10.1093/jn/138.6.1250S. PMID 18492865.
- ↑ "Health aspects of probiotics". IDrugs 6 (6): 573–80. 2003. PMID 12811680.
- ↑ Arya SC (1984). "Rotaviral infection and intestinal lactase level". J. Infect. Dis. 150 (5): 791. doi:10.1093/infdis/150.5.791. PMID 6436397.
- ↑ 41.0 41.1 "Routine laboratory testing data for surveillance of rotavirus hospitalizations to evaluate the impact of vaccination". Pediatr. Infect. Dis. J. 26 (10): 914–9. 2007. doi:10.1097/INF.0b013e31812e52fd. PMID 17901797.
- ↑ The Pediatric ROTavirus European CommitTee (PROTECT) (2006). "The paediatric burden of rotavirus disease in Europe". Epidemiol. Infect. 134 (5): 908–16. doi:10.1017/S0950268806006091. PMID 16650331.
- ↑ Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. 2009. p. 278. ISBN 978-0-12-375147-8.
- ↑ "Rotavirus typing methods and algorithms". Reviews in Medical Virology 14 (2): 71–82. 2004. doi:10.1002/rmv.411. PMID 15027000. PMC 7169166. https://zenodo.org/record/1229353.
- ↑ O'Ryan M (2007). "Rotarix (RIX4414): an oral human rotavirus vaccine". Expert Review of Vaccines 6 (1): 11–9. doi:10.1586/14760584.6.1.11. PMID 17280473.
- ↑ Matson DO (2006). "The pentavalent rotavirus vaccine, RotaTeq". Seminars in Pediatric Infectious Diseases 17 (4): 195–9. doi:10.1053/j.spid.2006.08.005. PMID 17055370.
- ↑ "MedTech от Ростеха: Назаров Александр Юрьевич рассказал о передовых медицинских разработках госкорпорации - Комиинформ". https://komiinform.ru/nt/7978.
- ↑ "Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines". The Journal of Infectious Diseases 200 (Suppl 1): S1–8. November 2009. doi:10.1086/605061. PMID 19817589.
- ↑ "Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data". The Pediatric Infectious Disease Journal 30 (1 Suppl): S56–60. January 2011. doi:10.1097/INF.0b013e3181fefdc0. PMID 21183842.
- ↑ "Review of rotavirus studies in Africa: 1976–2006". The Journal of Infectious Diseases 202 (Suppl): S23–33. September 2010. doi:10.1086/653554. PMID 20684708.
- ↑ Soares-Weiser, Karla; Bergman, Hanna; Henschke, Nicholas; Pitan, Femi; Cunliffe, Nigel (2019). "Vaccines for preventing rotavirus diarrhoea: vaccines in use". The Cochrane Database of Systematic Reviews 3: CD008521. doi:10.1002/14651858.CD008521.pub4. ISSN 1469-493X. PMID 30912133.
- ↑ "Influence of potential protective mechanisms on the development of live rotavirus vaccines". The Journal of Infectious Diseases 202 (Suppl): S72–9. September 2010. doi:10.1086/653549. PMID 20684721.
- ↑ "Global impact of rotavirus vaccines". Expert Review of Vaccines 9 (4): 395–407. April 2010. doi:10.1586/erv.10.17. PMID 20370550.
- ↑ Moszynski P (2011). "GAVI rolls out vaccines against child killers to more countries". BMJ (Clinical Research Ed.) 343: d6217. doi:10.1136/bmj.d6217. PMID 21957215.
- ↑ "Treatment of infectious diarrhea in children". Paediatr. Drugs 5 (3): 151–65. 2003. doi:10.2165/00128072-200305030-00002. PMID 12608880. https://www.semanticscholar.org/paper/9bfbf7e3253f2df877895a60d854eee2ce3e3277.
- ↑ Sachdev HP (1996). "Oral rehydration therapy". Journal of the Indian Medical Association 94 (8): 298–305. PMID 8855579.
- ↑ Ramig RF (August 2007). "Systemic rotavirus infection". Expert Review of Anti-infective Therapy 5 (4): 591–612. doi:10.1586/14787210.5.4.591. PMID 17678424.
- ↑ "Rotavirus gastroenteritis". Adv. Ther. 22 (5): 476–87. 2005. doi:10.1007/BF02849868. PMID 16418157.
- ↑ UNICEF/WHO (2009) "Diarrhoea: Why children are still dying and what can be done." Retrieved 23 May 2010
- ↑ Centers for Disease Control and Prevention (CDC) (October 2009). "Reduction in rotavirus after vaccine introduction—United States, 2000–2009". MMWR. Morbidity and Mortality Weekly Report 58 (41): 1146–9. PMID 19847149. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5841a2.htm. Retrieved 2009-12-20.
- ↑ "Recommendations for rotavirus vaccination: A worldwide perspective". Vaccine 28 (31): 5100–8. May 2010. doi:10.1016/j.vaccine.2010.04.108. PMID 20472032.
- ↑ "Rotavirus and severe childhood diarrhea". Emerging Infect. Dis. 12 (2): 304–6. 2006. doi:10.3201/eid1202.050006. PMID 16494759.
- ↑ "Hospital admissions attributable to rotavirus infection in England and Wales". J. Infect. Dis. 174 Suppl 1: S12–8. 1996. doi:10.1093/infdis/174.Supplement_1.S12. PMID 8752285.
- ↑ "Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis". International Journal of Epidemiology 38 (6): 1487–96. December 2009. doi:10.1093/ije/dyn260. PMID 19056806.
- ↑ "Seasonality and diversity of Group A rotaviruses in Europe". Acta Paediatrica Supplement 88 (426): 14–9. 1999. doi:10.1111/j.1651-2227.1999.tb14320.x. PMID 10088906.
- ↑ "Rotavirus infection in adults". The Lancet Infectious Diseases 4 (2): 91–9. February 2004. doi:10.1016/S1473-3099(04)00928-4. PMID 14871633.
- ↑ "A community waterborne gastroenteritis outbreak: evidence for rotavirus as the agent". American Journal of Public Health 74 (3): 263–5. 1984. doi:10.2105/AJPH.74.3.263. PMID 6320684.
- ↑ "Mutated G4P[8] rotavirus associated with a nationwide outbreak of gastroenteritis in Nicaragua in 2005". J. Clin. Microbiol. 45 (3): 990–7. 2007. doi:10.1128/JCM.01992-06. PMID 17229854.
- ↑ "An outbreak of rotavirus diarrhea among a non-immune, isolated South American Indian community". Am. J. Epidemiol. 113 (6): 703–10. 1981. doi:10.1093/oxfordjournals.aje.a113151. PMID 6263087.
- ↑ "Waterborne outbreak of rotavirus diarrhea in adults in China caused by a novel rotavirus". Lancet 1 (8387): 1139–42. 1984. doi:10.1016/S0140-6736(84)91391-6. PMID 6144874. https://www.semanticscholar.org/paper/a94e78791943e442a1651cd606ab2f3dc81088f0.
- ↑ "Investigation of an outbreak of adult diarrhea rotavirus in China". J. Infect. Dis. 160 (6): 948–53. 1989. doi:10.1093/infdis/160.6.948. PMID 2555422.
- ↑ "Group B rotaviruses similar to strain CAL-1, have been circulating in Western India since 1993". Epidemiol. Infect. 132 (4): 745–9. 2004. doi:10.1017/S0950268804002171. PMID 15310177.
- ↑ "Genetic analysis of group B human rotaviruses detected in Bangladesh in 2000 and 2001". J. Med. Virol. 72 (1): 149–55. 2004. doi:10.1002/jmv.10546. PMID 14635024.
- ↑ "Seroepidemiology of adult diarrhea rotavirus in China, 1977 to 1987". J. Clin. Microbiol. 27 (10): 2180–3. 1 October 1989. doi:10.1128/JCM.27.10.2180-2183.1989. PMID 2479654.
- ↑ "Studies on epidemic diarrhea of the new-born: Isolation of a Filtrable Agent Causing Diarrhea in Calves". Am. J. Public Health Nations Health 33 (12): 1451–4. 1943. doi:10.2105/AJPH.33.12.1451. PMID 18015921.
- ↑ "Diarrhea in gnotobiotic calves caused by the reovirus-like agent of human infantile gastroenteritis". Infect. Immun. 14 (2): 471–4. 1 August 1976. doi:10.1128/IAI.14.2.471-474.1976. PMID 184047.
- ↑ "The growth of the virus of epidemic diarrhoea of infant mice (EDIM) in organ cultures of intestinal epithelium". British Journal of Experimental Pathology 52 (4): 442–45. 1971. PMID 4998842.
- ↑ 78.0 78.1 "Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis in children, calves, piglets, mice, and foals". Infect. Immun. 14 (3): 804–10. 1 September 1976. doi:10.1128/IAI.14.3.804-810.1976. PMID 965097.
- ↑ 79.0 79.1 "The rotaviruses". Arch. Virol. 57 (1): 1–23. 1978. doi:10.1007/BF01315633. PMID 77663.
- ↑ "Relation between viruses from acute gastroenteritis of children and newborn calves". Lancet 2 (7872): 61–3. 1974. doi:10.1016/S0140-6736(74)91631-6. PMID 4137164.
- ↑ Matthews RE (1979). "Third report of the International Committee on Taxonomy of Viruses. Classification and nomenclature of viruses". Intervirology 12 (3–5): 129–296. doi:10.1159/000149081. PMID 43850.
- ↑ "The antigenic diversity of rotaviruses: significance to epidemiology and vaccine strategies". European Journal of Epidemiology 4 (1): 1–11. March 1988. doi:10.1007/BF00152685. PMID 2833405. https://www.semanticscholar.org/paper/a8005f9c2a3a069959c9794edfe617713d4d0a78.
- ↑ "Sequential passages of human rotavirus in MA-104 cells". Microbiol. Immunol. 25 (10): 1025–35. 1981. doi:10.1111/j.1348-0421.1981.tb00109.x. PMID 6273696.
- ↑ "Rotarix: a rotavirus vaccine for the world". Clinical Infectious Diseases 48 (2): 222–8. January 2009. doi:10.1086/595702. PMID 19072246.
- ↑ "Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR Recomm Rep 48 (RR–2): 1–20. 1999. PMID 10219046.
- ↑ Kapikian AZ (2001). "A rotavirus vaccine for prevention of severe diarrhoea of infants and young children: development, utilization and withdrawal". Gastroenteritis Viruses. Novartis Foundation Symposia. 238. 153–71; discussion 171–9. doi:10.1002/0470846534.ch10. ISBN 9780470846537.
- ↑ Bines, JE (2005). "Rotavirus vaccines and intussusception risk". Curr. Opin. Gastroenterol. 21 (1): 20–5. PMID 15687880. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0267-1379&volume=21&issue=1&spage=20.
- ↑ Bines J (2006). "Intussusception and rotavirus vaccines". Vaccine 24 (18): 3772–6. doi:10.1016/j.vaccine.2005.07.031. PMID 16099078.
- ↑ Dennehy PH (2008). "Rotavirus vaccines: an overview". Clin. Microbiol. Rev. 21 (1): 198–208. doi:10.1128/CMR.00029-07. PMID 18202442.
- ↑ "Meeting of the immunization Strategic Advisory Group of Experts, April 2009—conclusions and recommendations". Relevé Épidémiologique Hebdomadaire 84 (23): 220–36. June 2009. PMID 19499606.[1]
- ↑ 91.0 91.1 "Zoonotic aspects of rotaviruses". Veterinary Microbiology 140 (3–4): 246–55. January 2010. doi:10.1016/j.vetmic.2009.08.028. PMID 19781872. https://hal.archives-ouvertes.fr/hal-00556058/file/PEER_stage2_10.1016%252Fj.vetmic.2009.08.028.pdf.
- ↑ "Rotaviruses: diversity and zoonotic potential—a brief review". Berl. Munch. Tierarztl. Wochenschr. 120 (3–4): 108–12. 2007. PMID 17416132.
- ↑ "The zoonotic potential of rotavirus". J. Infect. 48 (4): 289–302. 2004. doi:10.1016/j.jinf.2004.01.018. PMID 15066329.
Further reading
- Ramig, RF (October 2004). "Pathogenesis of intestinal and systemic rotavirus infection". Journal of Virology 78 (19): 10213–20. doi:10.1128/JVI.78.19.10213-10220.2004. PMID 15367586.
External links
| Classification | |
|---|---|
| External resources |