Chemistry:Berkelium(III) oxide
From HandWiki
| |
| Names | |
|---|---|
| Other names
diberkelium trioxide, berkelium sequioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Bk2O3 | |
| Molar mass | 542 g·mol−1 |
| Appearance | yellow-green solid |
| Density | g/cm3 |
| Melting point | 1,920 °C (3,490 °F; 2,190 K) |
| insoluble | |
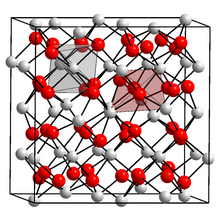
| Structure | |
| cubic | |
| Related compounds | |
Related compounds
|
Californium(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Berkelium(III) oxide is a binary inorganic compound of berkelium and oxygen with the chemical formula Bk2O3.[1]
Synthesis
Berkelium(III) oxide can be prepared from berkelium(IV) oxide by reduction with hydrogen:[2]
- 2BkO
2 + H
2 → Bk
2O
3 + H
2O
- 2BkO
Physical properties
The compound forms a yellow-green solid with a melting point of 1920 °C. It forms a body-centered cubic crystal lattice with a = 1088.0 ± 0.5 pm.[3][4]
Insoluble in water.[5]
References
- ↑ Seaborg, G. T.; Katz, Joseph J.; Morss, L. R. (6 December 2012) (in en). The Chemistry of the Actinide Elements: Volume 2. Springer Science & Business Media. p. 1004. ISBN 978-94-009-3155-8. https://books.google.com/books?id=ywDwCAAAQBAJ&dq=berkelium(III)+oxide+Bk2O3&pg=PA1004. Retrieved 11 April 2023.
- ↑ Sabry, Fouad (15 October 2022) (in en). Americium: Future space missions can be powered for up to 400 years. One Billion Knowledgeable. https://books.google.com/books?id=Z2yVEAAAQBAJ&dq=berkelium(III)+oxide&pg=PT77. Retrieved 11 April 2023.
- ↑ Peterson, J. R.; Cunningham, B. B. (1 September 1967). "Crystal structures and lattice parameters of the compounds of berkelium I. Berkelium dioxide and cubic berkelium sesquioxide" (in en). Inorganic and Nuclear Chemistry Letters 3 (9): 327–336. doi:10.1016/0020-1650(67)80037-0. ISSN 0020-1650. https://www.sciencedirect.com/science/article/abs/pii/0020165067800370. Retrieved 11 April 2023.
- ↑ Baybarz, R. D. (1 August 1968). "The berkelium oxide system" (in en). Journal of Inorganic and Nuclear Chemistry 30 (7): 1769–1773. doi:10.1016/0022-1902(68)80352-5. ISSN 0022-1902. https://www.sciencedirect.com/science/article/abs/pii/0022190268803525. Retrieved 11 April 2023.
- ↑ Schweitzer, George K.; Pesterfield, Lester L. (14 January 2010) (in en). The Aqueous Chemistry of the Elements. Oxford University Press. ISBN 978-0-19-539335-4. https://books.google.com/books?id=-oI8DwAAQBAJ&dq=berkelium(III)+oxide+Bk2O3&pg=PA406. Retrieved 11 April 2023.
 |

