Chemistry:Etirinotecan pegol
From HandWiki
Short description: Pharmaceutical drug
 | |
| Clinical data | |
|---|---|
| Trade names | Onzeald |
| Other names | NKTR-102 |
| Routes of administration | Intravenous infusion |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | none |
| Metabolites | irinotecan and its metabolites |
| Elimination half-life | 38 days |
| Excretion | mostly via kidneys |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C153H176N20O36[C8H16O4]n (n≈113) |
| Molar mass | 20,900–24,900 g/mol[1] |
Etirinotecan pegol (trade name Onzeald) is a drug developed by Nektar Therapeutics for the treatment of certain kinds of breast cancer with brain metastases. The European Medicines Agency refused to grant it a marketing authorisation in 2017.[2]
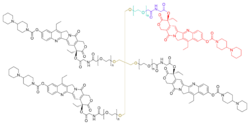
It works as a topoisomerase I inhibitor.[3] Chemically, it consists of four units of irinotecan (a topoisomerase I inhibitor in use since the late 1990s[4]) linked by carboxymethyl glycine and polyethylene glycol (PEG) chains to a central pentaerythritol ether, resulting in a much longer biological half-life (38 days) than that of irinotecan. It is formulated as a dihydrochloride and with 1.2 units of trifluoroacetate.[1]
References
- ↑ 1.0 1.1 "Onzeald: EPAR – Refusal public assessment report". European Medicines Agency. 2018-02-02. https://www.ema.europa.eu/documents/assessment-report/onzeald-epar-refusal-public-assessment-report_en.pdf.
- ↑ "Onzeald". European Medicines Agency. 2017-11-10. https://www.ema.europa.eu/en/medicines/human/EPAR/onzeald.
- ↑ "Health-related quality of life in patients with locally recurrent or metastatic breast cancer treated with etirinotecan pegol versus treatment of physician's choice: Results from the randomised phase III BEACON trial". European Journal of Cancer 76: 205–215. May 2017. doi:10.1016/j.ejca.2017.02.011. PMID 28360015.
- ↑ "Drug Approval Package: Camptosar (Irinotecan Hydrochloride) NDA# 20-571/S-008". https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/020571s008.cfm.
 |

