Chemistry:Temoporfin
From HandWiki
Short description: Chemical compound
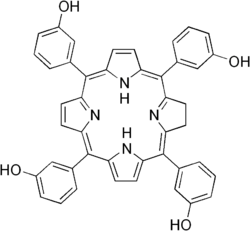 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C44H32N4O4 |
| Molar mass | 680.764 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Temoporfin (INN) is a photosensitizer (based on chlorin) used in photodynamic therapy for the treatment of squamous cell carcinoma of the head and neck[1] .[2] It is marketed in the European Union under the brand name Foscan. The U.S. Food and Drug Administration (FDA) declined to approve Foscan in 2000. The EU approved its use in June 2001.[3]
Good results were obtained in 21 of 35 patients treated in Germany.[4]
It is photoactivated at 652 nm[2] i.e. by red light.
Patients can remain photosensitive for several weeks after treatment.[2]
References
- ↑ "[Squamous cell carcinoma of the head and neck. Photodynamic therapy with Foscan]" (in de). Hno 56 (4): 402–409. April 2008. doi:10.1007/s00106-007-1573-1. PMID 17516041.
- ↑ 2.0 2.1 2.2 "Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy". Photochemistry and Photobiology 85 (5): 1053–1074. 2009. doi:10.1111/j.1751-1097.2009.00585.x. PMID 19682322.
- ↑ "Foscan approval saves Scotia's skin.". HighBeam. http://www.highbeam.com/doc/1P2-18794532.html.
- ↑ "Photodynamic therapy with meta-tetrahydroxyphenylchlorin (Foscan) in the management of squamous cell carcinoma of the head and neck: experience with 35 patients". European Archives of Oto-Rhino-Laryngology 266 (12): 1937–1944. December 2009. doi:10.1007/s00405-009-0947-2. PMID 19290535.
Further reading
- "Relationship between subcellular localisation of Foscan and caspase activation in photosensitised MCF-7 cells". British Journal of Cancer 96 (6): 944–51. March 2007. doi:10.1038/sj.bjc.6603631. PMID 17325708.
 |
