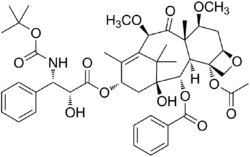
Chemistry:Cabazitaxel
 | |
| Clinical data | |
|---|---|
| Trade names | Jevtana |
| Other names | XRP-6258 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C45H57NO14 |
| Molar mass | 835.944 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid.[2] It is a microtubule inhibitor,[1] and the fourth taxane to be approved as a cancer therapy.[citation needed]
Cabazitaxel was developed by Sanofi-Aventis and was approved by the U.S. Food and Drug Administration (FDA) for the treatment of hormone-refractory prostate cancer in June 2010.[3][4][5] It is available as a generic medication.[6][7]
Medical uses
Cabazitaxel is indicated in combination with prednisone for the treatment of metastatic castration-resistant prostate cancer following docetaxel-based treatment.[1]
Mechanism of action
Taxanes enhance microtubule stabilization and inhibit cellular mitosis and division.[8] Moreover, taxanes prevent androgen receptor (AR) signaling by binding cellular microtubules and the microtubule-associated motor protein dynein, thus averting AR nuclear translocation.[9]
Clinical trials
In patients with metastatic castration-resistant prostate cancer (mCRPC), overall survival (OS) is markedly enhanced with cabazitaxel versus mitoxantrone after prior docetaxel treatment. FIRSTANA (ClinicalTrials.gov identifier: NCT01308567) assessed whether cabazitaxel 20 mg/m2 (C20) or 25 mg/m2 (C25) is superior to docetaxel 75 mg/m2 (D75) in terms of OS in patients with chemotherapy-naïve mCRPC. However, C20 and C25 did not demonstrate superiority for OS versus D75 in patients with chemotherapy-naïve mCRPC. Cabazitaxel and docetaxel demonstrated different toxicity profiles, and C20 showed the overall lowest toxicity.[10] In a phase III trial with 755 men for the treatment of castration-resistant prostate cancer, median survival was 15.1 months for patients receiving cabazitaxel versus 12.7 months for patients receiving mitoxantrone. Cabazitaxel was associated with more grade 3–4 neutropenia (81.7%) than mitoxantrone (58%).[11] Common adverse effects with cabazitaxel include neutropenia (including febrile neutropenia) and GIT side effects appeared mainly in diarrhea, whereas, neuropathy was rarely detected.[12]
Pharmacokinetics
Cabazitaxel administration causes a decrease in plasma concentrations showing triphasic kinetics: a mean half life (t1/2) of 2.6 min in the first phase, a mean t1/2 of 1.3 h in the second phase, and a mean t1/2 of 77.3 h in the third phase.[13]
Metabolism
Cabazitaxel is basically metabolized in the liver by [cytochrome P450 (CYP)3A4/5 > CYP2C8], which result in seven plasma metabolites and excreted 20 metabolites. During 14 days after administration, 80% of cabazitaxel is excreted: 76% in the feces and 3.7% as a renal excretion.[14]
References
- ↑ 1.0 1.1 1.2 "Jevtana- cabazitaxel kit". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de3d9c26-572b-4ea4-9b2d-dd58a2b3e8fa.
- ↑ "Cabazitaxel". NCI Drug Dictionary. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. February 2, 2011. http://www.cancer.gov/drugdictionary/?CdrID=534131.
- ↑ "Drug Approval Package: Jevtana (Cabazitaxel) NDA #201023". July 8, 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/201023s000TOC.cfm.
- ↑ "Jevtana (cabazitaxel) Injection Approved by U.S. FDA After Priority Review" (Press release). Sanofi Aventis. June 17, 2010. Retrieved December 30, 2021 – via PR Newswire.
- ↑ "Jevtana (cabazitaxel) Injection Approved by U.S. FDA After Priority Review - Jun 17, 2010" (Press release). Sanofi. June 17, 2010. Retrieved December 30, 2021.
- ↑ "Cabazitaxel: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=207949.
- ↑ "First Generic Drug Approvals". October 17, 2022. https://www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals.
- ↑ "Microtubules as a target for anticancer drugs". Nature Reviews. Cancer 4 (4): 253–65. April 2004. doi:10.1038/nrc1317. PMID 15057285.
- ↑ "Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer". Cancer Research 71 (18): 6019–29. September 2011. doi:10.1158/0008-5472.CAN-11-1417. PMID 21799031.
- ↑ "Cabazitaxel Versus Docetaxel As First-Line Therapy for Patients With Metastatic Castration-Resistant Prostate Cancer: A Randomized Phase III Trial-FIRSTANA". Journal of Clinical Oncology 35 (28): 3189–3197. October 2017. doi:10.1200/JCO.2016.72.1068. PMID 28753384.
- ↑ "Cabazitaxel Effective for Hormone Refractory Prostate Cancer After Failure of Taxotere". http://professional.cancerconsultants.com/oncology_main_news.aspx?id=44709.[yes|permanent dead link|dead link}}]
- ↑ "Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer". Drug Design, Development and Therapy 5: 117–24. March 2011. doi:10.2147/DDDT.S13029. PMID 21448449.
- ↑ "Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors". Clinical Cancer Research 15 (2): 723–30. January 2009. doi:10.1158/1078-0432.CCR-08-0596. PMID 19147780.
- ↑ "The role of cabazitaxel in the treatment of metastatic castration-resistant prostate cancer". Therapeutic Advances in Urology 6 (3): 97–104. June 2014. doi:10.1177/1756287214528557. PMID 24883107.
External links
- "Cabazitaxel". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/cabazitaxel.
- "Cabazitaxel Accord 20 mg/mL concentrate for solution infusion: Risk of medication errors and mix-up with Jevtana (60 mg/1.5 mL) solvent infusion". October 28, 2020. https://www.ema.europa.eu/en/medicines/dhpc/cabazitaxel-accord-20-mgml-concentrate-solution-infusion-risk-medication-errors-mix-jevtana-60-mg15.
- Clinical trial number NCT00417079 for "XRP6258 Plus Prednisone Compared to Mitoxantrone Plus Prednisone in Hormone Refractory Metastatic Prostate Cancer (TROPIC)" at ClinicalTrials.gov
- Clinical trial number NCT01308580 for "Cabazitaxel at 20 mg/m² Compared to 25 mg/m² With Prednisone for the Treatment of Metastatic Castration Resistant Prostate Cancer (PROSELICA)" at ClinicalTrials.gov
- Clinical trial number NCT02485691 for "Cabazitaxel Versus the Switch to Alternative AR-targeted Agent (Enzalutamide or Abiraterone) in Metastatic Castration-resistant Prostate Cancer (mCRPC) Patients Previously Treated With Docetaxel and Who Rapidly Failed a Prior AR-targeted Agent (CARD)" at ClinicalTrials.gov
 |
