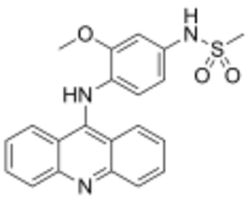
Chemistry:Amsacrine
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 96 to 98% |
| Elimination half-life | 8–9 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H19N3O3S |
| Molar mass | 393.46 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Amsacrine (synonyms: m-AMSA, acridinyl anisidide) is an antineoplastic agent.
It has been used in acute lymphoblastic leukemia.[1]
Mechanism
Its planar fused ring system can intercalate into the DNA of tumor cells, thereby altering the major and minor groove proportions. These alterations to DNA structure inhibit both DNA replication and transcription by reducing association between the affected DNA and: DNA polymerase, RNA polymerase and transcription factors.
Amsacrine also expresses topoisomerase inhibitor activity, specifically inhibiting topoisomerase II.[2] In contrast, the structurally similar o-AMSA differing in the position of the methoxy substituent group on the anilino-ring have little ability to poison topoisomerase II despite its intercalative behavior, suggesting that intercalation of the molecule in itself is insufficient to trap topoisomerase II as a covalent complex on DNA.[3][4][5]
References
- ↑ "Amsacrine combined with etoposide and high-dose methylprednisolone as salvage therapy in acute lymphoblastic leukemia in children". Haematologica 90 (12): 1701–3. December 2005. PMID 16330449.
- ↑ "Amsacrine as a Topoisomerase II Poison: Importance of Drug-DNA Interactions". Biochemistry 51 (8): 1730–1739. February 2012. doi:10.1021/bi201159b. PMID 22304499.
- ↑ "Thermodynamics of the interactions of m-AMSA and o-AMSA with nucleic acids: influence of ionic strength and DNA base composition". Nucleic Acids Research 17 (23): 9933–46. December 1989. doi:10.1093/nar/17.23.9933. PMID 2602146.
- ↑ "Mutagenicity of m-AMSA and o-AMSA in mammalian cells due to clastogenic mechanism: possible role of topoisomerase". Mutagenesis 2 (5): 349–55. September 1987. doi:10.1093/mutage/2.5.349. PMID 2830452.
- ↑ "Targeting DNA topoisomerase II in cancer chemotherapy". Nature Reviews. Cancer 9 (5): 338–50. May 2009. doi:10.1038/nrc2607. PMID 19377506.
 |
