Chemistry:Lurtotecan
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
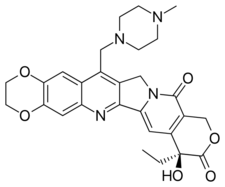
(8S)-8-Ethyl-8-hydroxy-15-[(4-methylpiperazin-1-yl)methyl]-2,3-dihydro-11H-[1,4]dioxino[2,3-g]pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-9,12(8H,14H)-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H30N4O6 | |
| Molar mass | 518.561 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Lurtotecan is a semi-synthetic analog of camptothecin with antineoplastic activity. Liposomal lurtotecan was in clinical trials as a treatment for topotecan-resistant ovarian cancer,[1] but was discontinued.[2]
Synthesis
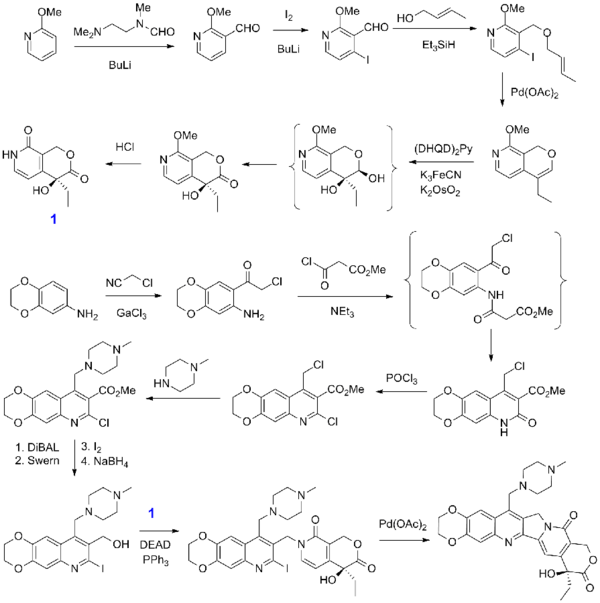
- Heck reaction
- Mitsunobu reaction
- Potassium osmate
- Sharpless asymmetric dihydroxylation
- Swern oxidation
References
- ↑ Seiden, MV; Muggia, F; Astrow, A; Matulonis, U; Campos, S; Roche, M; Sivret, J; Rusk, J et al. (April 2004). "A Phase II Study of Liposomal Lurtotecan (OSI-211) in Patients with Topotecan Resistant Ovarian Cancer". Gynecologic Oncology 93 (1): 229–32. doi:10.1016/j.ygyno.2003.12.037. PMID 15047241.
- ↑ "Liposomal lurtotecan (OSI 211) on AdisInsight". Springer International Publishing AG. http://adisinsight.springer.com/drugs/800010410.
- ↑ Fang, F. G.; Bankston, D. D.; Huie, E. M.; Ross Johnson, M.; Kang, M. C.; Lehoullier, C. S.; Lewis, G. C.; Lovelace, T. C. et al. (1997). "Convergent catalytic asymmetric synthesis of camptothecin analog GI147211C". Tetrahedron 53 (32): 10953. doi:10.1016/S0040-4020(97)00357-8.
 |
