Chemistry:Vinflunine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
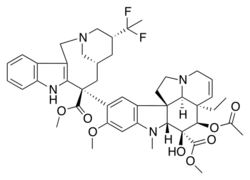
| Formula | C45H54F2N4O8 |
| Molar mass | 816.944 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Vinflunine (INN, trade name Javlor) is a novel fluorinated vinca alkaloid derivative[2] undergoing research for the treatment of bladder cancer. It was originally discovered by the team of the Professor Jean-Claude Jacquesy (UMR CNRS 6514 – Poitiers University),[3] developed by Laboratoires Pierre Fabre and was licensed to Bristol-Myers Squibb for development in certain countries, including the United States .
On November 23, 2007, Pierre Fabre Medicament and Bristol-Myers Squibb announced that they were terminating their license agreement for the development of vinflunine, and that Pierre Fabre were continuing "discussions with regulatory authorities and plan to file for the registration of vinflunine for bladder cancer in the first quarter of 2008."[4]
Approvals and indications
(As of 2012), vinflunine was registered for use in Australia for "advanced or metastatic transitional cell carcinoma of the urothelial tract after failure of a prior platinum containing regimen," but is not covered under the Pharmaceutical Benefits Scheme.[5]
(As of 2016), vinflunine was the only commercially-approved agent in some countries for salvage therapy of urothelial carcinoma, (with approval based on the results of a phase III trial), with a reported median OS of about 6 months.[6][7]
Clinical trials
It has undergone a phase III clinical trial for advanced transitional cell carcinoma of the urothelial tract.[7]
References
- ↑ "Javlor EPAR". https://www.ema.europa.eu/en/medicines/human/EPAR/javlor.
- ↑ "Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid". Biochemical Pharmacology 55 (5): 635–48. March 1998. doi:10.1016/S0006-2952(97)00505-4. PMID 9515574.
- ↑ "Vinca Alkaloids in Superacidic Media: A Method for Creating a New Family of Antitumor Derivatives". J. Am. Chem. Soc. 119 (36): 8576–8577. 1997. doi:10.1021/ja971864w.
- ↑ "Bristol-Myers Squibb and Pierre Fabre Provide Update On Vinflunine Development Status" (Press release). Bristol-Myers Squibb. November 23, 2007. Archived from the original on May 28, 2008. Retrieved June 27, 2008.
- ↑ "Vinflunine, solution concentration for I.V. infusion, 50 mg in 2 mL and 250 mg in 10 mL (as ditartrate), Javlor® – November 2011". Pharmaceutical Benefits Scheme. March 16, 2012. http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/2011-11/pbac-psd-vinflunine-nov11.
- ↑ "Systemic Therapy for Urothelial Carcinoma: Is a Renaissance Around the Corner?". Oncology 30 (6): 580–3, 588. June 2016. PMID 27306713. http://www.cancernetwork.com/oncology-journal/systemic-therapy-urothelial-carcinoma-renaissance-around-corner.
- ↑ 7.0 7.1 "Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract". Journal of Clinical Oncology 27 (27): 4454–61. September 2009. doi:10.1200/JCO.2008.20.5534. PMID 19687335.
External links
- "Vinflunine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/vinflunine.
 |

