Chemistry:Altretamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Hexalen |
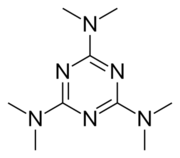
| Other names | 2,4,6-Tris(dimethylamino)-1,3,5-triazine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601200 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral (capsules) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 94% |
| Metabolism | Extensive liver |
| Metabolites | Pentamethylmelamine, tetramethylmelamine |
| Elimination half-life | 4.7–10.2 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C9H18N6 |
| Molar mass | 210.285 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Altretamine (trade name Hexalen), also called hexamethylmelamine, is an antineoplastic agent. It was approved by the U.S. FDA in 1990.
Uses
It is indicated for use as a single agent in the palliative treatment of patients with persistent or recurrent ovarian cancer following first-line therapy with cisplatin and/or alkylating agent-based combination.[1]
It is not considered a first-line treatment,[2] but it can be useful as salvage therapy.[3] It also has the advantage of being less toxic than other drugs used for treating refractory ovarian cancer.[4]
Mechanism
The precise mechanism by which altretamine exerts its anti-cancer effect is unknown but it is classified by MeSH as an alkylating antineoplastic agent.[5]
This unique structure is believed to damage tumor cells through the production of the weakly alkylating species formaldehyde, a product of CYP450-mediated N-demethylation. Administered orally, altretamine is extensively metabolized on first pass, producing primarily mono- and didemethylated metabolites. Additional demethylation reactions occur in tumor cells, releasing formaldehyde in situ before the drug is excreted in the urine. The carbinolamine (methylol) intermediates of CYP450-mediated metabolism also can generate electrophilic iminium species that are capable of reacting covalently with DNA guanine and cytosine residues as well as protein. Iminium-mediated DNA cross-linking and DNA-protein interstrand cross-linking, mediated through both the iminium intermediate and formaldehyde, have been demonstrated, although the significance of DNA cross-linking on altretamine antitumor activity is uncertain.[6]
Side effects
Side effects include nausea, vomiting, anemia and peripheral sensory neuropathy.[7]
Interactions
Combination with pyridoxine (vitamin B6) decreases neurotoxicity but has been found to reduce the effectiveness of an altretamine/cisplatin regime.[8] MAO inhibitor can cause severe orthostatic hypotension when combined with altretamine; and cimetidine can increase its elimination half-life and toxicity.[7]
See also
References
- ↑ "Hexalen (altretamine) Capsule. Human Prescription Drug Label". Eisai Inc.. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8456a8db-a7f6-4bc0-86be-e9c8c374140b.
- ↑ "Altretamine (hexamethylmelamine) in the treatment of platinum-resistant ovarian cancer: a phase II study". Gynecologic Oncology 88 (2): 118–122. February 2003. doi:10.1016/S0090-8258(02)00103-8. PMID 12586589.
- ↑ "Oral altretamine used as salvage therapy in recurrent ovarian cancer". Gynecologic Oncology 92 (1): 368–371. January 2004. doi:10.1016/j.ygyno.2003.09.017. PMID 14751188.
- ↑ "Altretamine is an effective palliative therapy of patients with recurrent epithelial ovarian cancer". Japanese Journal of Clinical Oncology 31 (2): 69–73. February 2001. doi:10.1093/jjco/hye012. PMID 11302345.
- ↑ "Clinical pharmacokinetics of altretamine". Clinical Pharmacokinetics 28 (6): 439–448. June 1995. doi:10.2165/00003088-199528060-00002. PMID 7656502.
- ↑ Foye's Principles of Medicinal Chemistry (6th ed.). Philadelphia: Lippincott Williams & Wilkins. 2008. ISBN 978-0-7817-6879-5.
- ↑ 7.0 7.1 "Altretamine Monograph". Drugs.com. https://www.drugs.com/monograph/altretamine.html.
- ↑ "Hexamethylmelamine and low or moderate dose cisplatin with or without pyridoxine for treatment of advanced ovarian carcinoma: a study of the Eastern Cooperative Oncology Group". Cancer Investigation 10 (1): 1–9. 1992. doi:10.3109/07357909209032783. PMID 1735009.
 |
