Chemistry:Halometasone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Sicorten |
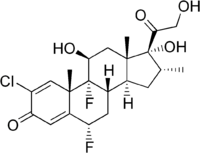
| Other names | (6S,8S,9R,10S,11S,13S,16R,17R)-2-chloro-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxy-acetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Topical |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H27ClF2O5 |
| Molar mass | 444.90 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Halometasone is a potent (Group III) synthetic tri-halogenated corticosteroid for topical application possessing pronounced anti-inflammatory, antiexudative, antiepidermoplastic, antiallergic, and antipruritic properties. It has been approved in many European countries including Spain, Germany, Switzerland, Austria, Netherlands, Belgium, and Portugal and other regions such as China, Hong Kong, Turkey, Israel, South Africa and India.
It has been used to treat chronic psoriasis vulgaris[1] and non-infected acute eczematous dermatoses (eczema).[2] One study demonstrated that 0.05% halometasone cream was more effective than 0.05% betamethasone cream in treating dermatitis, though both were well tolerated, with no systemic adverse effects reported.[3]
References
- ↑ "Halometasone cream by day and halometasone ointment at night for the treatment of patients with chronic psoriasis vulgaris". The Journal of International Medical Research 11 (Suppl 1): 31–3. 1983. PMID 6339290.
- ↑ "An overview of international clinical trials with halometasone cream". The Journal of International Medical Research 11 (Suppl 1): 1–7. 1983. PMID 6339286.
- ↑ "[Comparative clinical trial of a new trihalogenated dermatocorticoid (halometasone) versus betamethasone dipropionate]". Zeitschrift für Hautkrankheiten 58 (4): 230–7. February 1983. PMID 6342285.
 |

